Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin
- PMID: 35630729
- PMCID: PMC9145333
- DOI: 10.3390/molecules27103254
Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin
Abstract
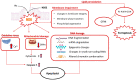
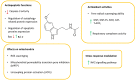
Cryoprotective and cytoprotective agents (Cytoprotective Agents) are fundamental components of the cryopreservation process. This review presents the essentials of the cryopreservation process by examining its drawbacks and the role of cytoprotective agents in protecting cell physiology. Natural cryoprotective and cytoprotective agents, such as antifreeze proteins, sugars and natural deep eutectic systems, have been compared with synthetic ones, addressing their mechanisms of action and efficacy of protection. The final part of this article focuses melatonin, a hormonal substance with antioxidant properties, and its emerging role as a cytoprotective agent for somatic cells and gametes, including ovarian tissue, spermatozoa and spermatogonial stem cells.
Keywords: DMSO; antioxidant; cryopreservation; cytoprotection; gametes; melatonin; stem cell.
Conflict of interest statement
S.C. and M.P. are employees of Angelantoni Life Science S.r.l., Italy. The rest of the authors declare no conflicts of interest.
Figures

References
-
- Torquato P., Giusepponi D., Bartolini D., Barola C., Marinelli R., Sebastiani B., Galarini R., Galli F. Pre-analytical monitoring and protection of oxidizable lipids in human plasma (vitamin E and omega-3 and omega-6 fatty acids): An update for redox-lipidomics methods. Free Radic. Biol. Med. 2021;176:142–148. doi: 10.1016/j.freeradbiomed.2021.09.012. - DOI - PubMed
-
- Pegg D.E. Principles of cryopreservation. Methods Mol. Biol. 2007;368:39–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

