Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions
- PMID: 35630892
- PMCID: PMC9147655
- DOI: 10.3390/nano12101672
Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions
Abstract
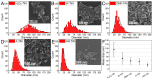
Owing to a strong photothermal response in the near-IR spectral range and very low toxicity, titanium nitride (TiN) nanoparticles (NPs) synthesized by pulsed laser ablation in liquids (PLAL) present a novel appealing object for photo-induced therapy of cancer, but the properties of these NPs still require detailed investigation. Here, we have elaborated methods of femtosecond laser ablation from the TiN target in a variety of liquid solutions, including acetonitrile, dimethylformamide, acetone, water, and H2O2, to synthesize TiN NPs and clarify the effect of liquid type on the composition and properties of the formed NPs. The ablation in all solvents led to the formation of spherical NPs with a mean size depending on the liquid type, while the composition of the NPs ranged from partly oxidized TiN to almost pure TiO2, which conditioned variations of plasmonic peak in the region of relative tissue transparency (670-700 nm). The degree of NP oxidation depended on the solvent, with much stronger oxidation for NPs prepared in aqueous solutions (especially in H2O2), while the ablation in organic solvents resulted in a partial formation of titanium carbides as by-products. The obtained results contribute to better understanding of the processes in reactive PLAL and can be used to design TiN NPs with desired properties for biomedical applications.
Keywords: EDX; XPS; laser ablation in aqueous and organic solutions; pulsed laser ablation in liquids; titanium nitride nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Toth L.E. In: Transition Metal Carbides and Nitrides. Margrave J.L., editor. Academic Press; New York, NY, USA: 1971.
-
- Avasarala B., Haldar P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Electrochim. Acta. 2010;55:9024–9034. doi: 10.1016/j.electacta.2010.08.035. - DOI
-
- Patsalas P., Kalfagiannis N., Kassavetis S. Optical Properties and Plasmonic Performance of Titanium Nitride. Materials. 2015;8:3128–3154. doi: 10.3390/ma8063128. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

