The Nanofibrous CaO Sorbent for CO2 Capture
- PMID: 35630899
- PMCID: PMC9146495
- DOI: 10.3390/nano12101677
The Nanofibrous CaO Sorbent for CO2 Capture
Abstract
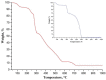
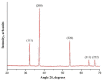
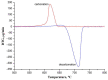
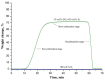
The nanofibrous CaO sorbent for high-temperature CO2 capture was fabricated by the calcination of electrospun composite filaments containing calcium acetylacetonate and polyacrylonitrile as a calcium-oxide precursor and a binder polymer, respectively. The calcination was carried out in air to prevent PAN carbonization and to obtain pure CaO nanofibers. The resulting mats of CaO nanofibers with the average diameter of 130 nm were characterized by a specific surface area of 31 m2/g, a CO2-uptake capacity of 16.4 mmol/g at the carbonation temperature of 618 °C, a hardness of 1.87 MPa, and the indentation Young's modulus of 786 MPa. The low decarbonation temperature makes the fabricated sorbent promising, for example, for the calcium-looping technology of CO2 removal from the hot exhaust gases of fossil-fueled power plants.
Keywords: CO2-uptake capacity; CaO nanofibers; chemisorption; electrospinning; mechanical properties; microstructure; phase composition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gao Y., Gao X., Zhang X. The 2 °C Global Temperature Target and the Evolution of the Long-Term Goal of Addressing Climate Change—From the United Nations Framework Convention on Climate Change to the Paris Agreement. Engineering. 2017;3:272–278. doi: 10.1016/J.ENG.2017.01.022. - DOI
-
- Gür T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2021;89:100965. doi: 10.1016/j.pecs.2021.100965. - DOI
-
- Buckingham J., Reina T.R., Duyar M.S. Recent advances in carbon dioxide capture for process intensification. Carbon Capture Sci. Technol. 2022;2:100031. doi: 10.1016/j.ccst.2022.100031. - DOI
-
- Sun H., Wu C., Shen B., Zhang X., Zhang Y., Huang J. Progress in the development and application of CaO-based adsorbents for CO2 capture–a review. Mater. Today Sustain. 2018;1–2:1–27. doi: 10.1016/j.mtsust.2018.08.001. - DOI
-
- Hu Y., Liu W., Yang Y., Qu M., Li H. CO2 capture by Li4SiO4 sorbents and their applications: Current developments and new trends. Chem. Eng. J. 2018;359:604–625. doi: 10.1016/j.cej.2018.11.128. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

