Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health
- PMID: 35631237
- PMCID: PMC9147914
- DOI: 10.3390/nu14102096
Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health
Abstract
The colon harbours a dynamic and complex community of microorganisms, collectively known as the gut microbiota, which constitutes the densest microbial ecosystem in the human body. These commensal gut microbes play a key role in human health and diseases, revealing the strong potential of fine-tuning the gut microbiota to confer health benefits. In this context, dietary strategies targeting gut microbes to modulate the composition and metabolic function of microbial communities are of increasing interest. One such dietary strategy is the use of prebiotics, which are defined as substrates that are selectively utilised by host microorganisms to confer a health benefit. A better understanding of the metabolic pathways involved in the breakdown of prebiotics is essential to improve these nutritional strategies. In this review, we will present the concept of prebiotics, and focus on the main sources and nature of these components, which are mainly non-digestible polysaccharides. We will review the breakdown mechanisms of complex carbohydrates by the intestinal microbiota and present short-chain fatty acids (SCFAs) as key molecules mediating the dialogue between the intestinal microbiota and the host. Finally, we will review human studies exploring the potential of prebiotics in metabolic diseases, revealing the personalised responses to prebiotic ingestion. In conclusion, we hope that this review will be of interest to identify mechanistic factors for the optimization of prebiotic-based strategies.
Keywords: carbohydrate metabolism; gut microbiota; health and well-being; personalised nutrition; prebiotics; short-chain fatty acids.
Conflict of interest statement
The authors declare that C.B.-F. has worked as an employee of Yoplait France—General Mills during the conduct of this study, as part of a CIFRE contract with Association Nationale de la Recherche et de la Technologie (2018/1183). The company had no role in the writing of this review or the decision to submit it for publication. The other authors declare no competing interests.
Figures
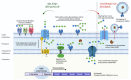
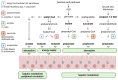

References
-
- Aron-Wisnewsky J., Warmbrunn M.V., Nieuwdorp M., Clément K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology. 2021;160:573–599. doi: 10.1053/j.gastro.2020.10.057. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

