Does Bypass of the Proximal Small Intestine Impact Food Intake, Preference, and Taste Function in Humans? An Experimental Medicine Study Using the Duodenal-Jejunal Bypass Liner
- PMID: 35631283
- PMCID: PMC9145649
- DOI: 10.3390/nu14102141
Does Bypass of the Proximal Small Intestine Impact Food Intake, Preference, and Taste Function in Humans? An Experimental Medicine Study Using the Duodenal-Jejunal Bypass Liner
Abstract
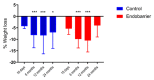
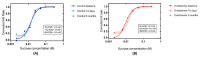
The duodenal-jejunal bypass liner (Endobarrier) is an endoscopic treatment for obesity and type 2 diabetes mellitus (T2DM). It creates exclusion of the proximal small intestine similar to that after Roux-en-Y Gastric Bypass (RYGB) surgery. The objective of this study was to employ a reductionist approach to determine whether bypass of the proximal intestine is the component conferring the effects of RYGB on food intake and sweet taste preference using the Endobarrier as a research tool. A nested mechanistic study within a large randomised controlled trial compared the impact of lifestyle modification with vs. without Endobarrier insertion in patients with obesity and T2DM. Forty-seven participants were randomised and assessed at several timepoints using direct and indirect assessments of food intake, food preference and taste function. Patients within the Endobarrier group lost numerically more weight compared to the control group. Using food diaries, our results demonstrated similar reductions of food intake in both groups. There were no significant differences in food preference and sensory, appetitive reward, or consummatory reward domain of sweet taste function between groups or changes within groups. In conclusion, the superior weight loss seen in patients with obesity and T2DM who underwent the Endobarrier insertion was not due to a reduction in energy intake or change in food preferences.
Keywords: Endobarrier; eating behaviour; food preferences; obesity; taste function.
Conflict of interest statement
A.R. received travel fees support from GI Dynamics. A.D.M. has received honoraria for presentations and advisory board contribution by Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Johnson & Johnson and research grant funding from Fractyl. A.P.G. reports funding supported by UK Medical Research Council and Wellcome Trust, outside of the submitted work, was on a Data Safety Monitoring Board for Novo Nordisk, and has received honoraria for presentations and advisory board contribution by Janssen, Pfizer, Novo Nordisk, Zafgen, Soleno Therapeutics Inc, and Millendo Theapeutics Inc, and Merck. C.W.R. is a member of scientific advisory board for Herbalife, GI Dynamics, NovoNordisk, Keyron, Sanofi, has provided ad hoc consulting for Ethicon and Fractyl, occasional speaking engagement for MSD, Boehringer Ingelheim and Lilly. J.P.T. received travel fees support from GI Dynamics. W.A. has received honoraria for presentations and educational grants from Novo Nordisk. The rest of the authors report no conflicts of interest. “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
Figures




References
-
- Kapoor N., al Najim W., Menezes C., Price R.K., O’Boyle C., Bodnar Z., Spector A.C., Docherty N.G., le Roux C.W. A Comparison of Total Food Intake at a Personalised Buffet in People with Obesity, before and 24 Months after Roux-en-Y-Gastric Bypass Surgery. Nutrients. 2021;13:3873. doi: 10.3390/nu13113873. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

