Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function
- PMID: 35631293
- PMCID: PMC9144241
- DOI: 10.3390/nu14102152
Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function
Abstract
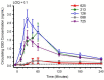
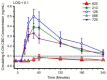
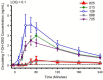
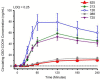
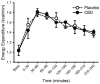
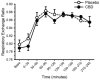
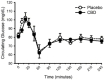
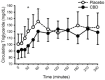
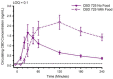
Cannabidiol (CBD) is widely available and marketed as having therapeutic properties. Over-the-counter CBD is unregulated, many of the therapeutic claims lack scientific support, and controversy exists as to the safety of CBD-liver interaction. The study aims were to compare the pharmacokinetics of commercial CBD and CBD metabolites following the ingestion of five different CBD formulations, determine the influence of CBD on food induced thermogenesis, determine the influence of food on CBD pharmacokinetics, and determine the influence of CBD on markers of liver function. Fourteen males (body mass index ≥ 25 kg/m2) were studied in a placebo-controlled, randomized, crossover design. On five occasions, different CBD formulations were ingested (one per visit). On two additional occasions, CBD or placebo was ingested following a meal. CBD servings were standardized to 30 mg. Considerable pharmacokinetic variability existed between formulations; this pharmacokinetic variability transferred to several of the metabolites. CBD did not influence food induced thermogenesis but did favorably modify early insulin and triglyceride responses. Food appreciably altered the pharmacokinetics of CBD. Finally, CBD did not evoke physiologically relevant changes in markers of liver function. Collectively, these data suggest that consumers should be aware of the appreciable pharmacokinetic differences between commercial CBD formulations, CBD is unlikely to influence the caloric cost of eating but may prove to be of some benefit to initial metabolic responses, consuming CBD with food alters the dynamics of CBD metabolism and increases systemic availability, and low-dose CBD probably does not represent a risk to normal liver function.
Keywords: cannabinoid; cannabis; energy expenditure; insulin; metabolism; pharmacodynamics; thermogenesis; triglyceride.
Conflict of interest statement
The authors declare no conflict of interest. The funder, Caliper Foods, had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Williams N.N.B., Ewell T.R., Abbotts K.S.S., Harms K.J., Woelfel K.A., Dooley G.P., Weir T.L., Bell C. Comparison of Five Oral Cannabidiol Preparations in Adult Humans: Pharmacokinetics, Body Composition, and Heart Rate Variability. Pharmaceuticals. 2021;14:35. doi: 10.3390/ph14010035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

