Poly (N-Vinylcaprolactam-Grafted-Sodium Alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation
- PMID: 35631636
- PMCID: PMC9143242
- DOI: 10.3390/pharmaceutics14051050
Poly (N-Vinylcaprolactam-Grafted-Sodium Alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation
Retraction in
-
RETRACTED: Khan et al. Poly (N-vinylcaprolactam-grafted-sodium alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation. Pharmaceutics 2022, 14, 1050.Pharmaceutics. 2024 Jan 22;16(1):149. doi: 10.3390/pharmaceutics16010149. Pharmaceutics. 2024. PMID: 38276522 Free PMC article.
Abstract
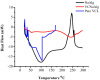
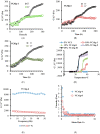
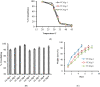
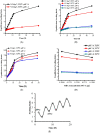
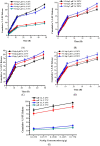
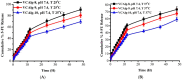
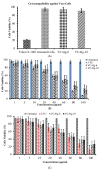
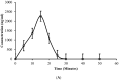
This study was aimed to develop novel in situ forming gels based on N-vinylcaprolactam, sodium alginate, and N,N-methylenebisacrylamide. The in situ Poly (NVRCL-g-NaAlg) gels were developed using the cold and free radical polymerization method. The structure formation, thermal stability, and porous nature of gels was confirmed by FTIR, NMR, DSC, TGA, and SEM. The tunable gelation temperature was evaluated by tube titling and rheological analysis. Optical transmittance showed that all formulations demonstrated phase transition around 33 °C. The swelling and release profile showed that gels offered maximum swelling and controlled 5-FU release at 25 °C and pH (7.4), owing to a relaxed state. Porosity and mesh size showed an effect on swelling and drug release. The in vitro degradation profile demonstrated a controlled degradation rate. An MTT assay confirmed that formulations are safe tested against Vero cells. In vitro cytotoxicity showed that 5-FU loaded gels have controlled cytotoxic potential against HeLa and MCF-7 cells (IC50 = 39.91 µg/mL and 46.82 µg/mL) compared to free 5-FU (IC50 = 50.52 µg/mL and 53.58 µg/mL). Histopathological study demonstrated no harmful effects of gels on major organs. The in vivo bioavailability in rabbits showed a controlled release in gel form (Cmax, 1433.59 ± 45.09 ng/mL) compared to a free drug (Cmax, 2263.31 ± 13.36 ng/mL) after the subcutaneous injection.
Keywords: 5-FU in situ depot; MTT assay; N-(vinylcaprolactam); anticancer drugs; chemical grafting; hydrogels; pharmacokinetics; rheology; sodium alginate.
Conflict of interest statement
No potential conflicts of interest were reported by the authors.
Figures











References
-
- Langer R., Peppas N.A. Advances in biomaterials, drug delivery and bionanotechnology. AIChE J. 2003;49:2990–3006. doi: 10.1002/aic.690491202. - DOI
-
- Rao K.M., Rao K.S.V.K., Sudhakar P., Rao K.C., Subha M.C.S. Synthesis and Characterization of biodegradable Poly (Vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013;3:061–069.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

