Functional Characterization of MtrGSTF7, a Glutathione S-Transferase Essential for Anthocyanin Accumulation in Medicago truncatula
- PMID: 35631744
- PMCID: PMC9147808
- DOI: 10.3390/plants11101318
Functional Characterization of MtrGSTF7, a Glutathione S-Transferase Essential for Anthocyanin Accumulation in Medicago truncatula
Abstract
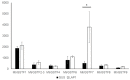
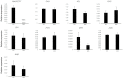
Flavonoids are essential compounds widespread in plants and exert many functions such as defence, definition of organ colour and protection against stresses. In Medicago truncatula, flavonoid biosynthesis and accumulation is finely regulated in terms of tissue specificity and induction by external factors, such as cold and other stresses. Among flavonoids, anthocyanin precursors are synthesised in the cytoplasm, transported to the tonoplast, then imported into the vacuole for further modifications and storage. In the present work, we functionally characterised MtrGSTF7, a phi-class glutathione S-transferase involved in anthocyanin transport to the tonoplast. The mtrgstf7 mutant completely lost the ability to accumulate anthocyanins in leaves both under control and anthocyanin inductive conditions. On the contrary, this mutant showed an increase in the levels of soluble proanthocyanidins (Pas) in their seeds with respect to the wild type. By complementation and expression data analysis, we showed that, differently from A. thaliana and similarly to V. vinifera, transport of anthocyanin and proanthocyanidins is likely carried out by different GSTs belonging to the phi-class. Such functional diversification likely results from the plant need to finely tune the accumulation of diverse classes of flavonoids according to the target organs and developmental stages.
Keywords: Medicago truncatula; Tnt1 insertional mutants; anthocyanin transport; flavonoids; gluthatione S-transferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Antony U., Chandra T.S. Antinutrient Reduction and Enhancement in Protein, Starch, and Mineral Availability in Fermented Flour of Finger Millet (Eleusine coracana) J. Agric. Food Chem. 1998;46:2578–2582. doi: 10.1021/jf9706639. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

