In Vitro Anti-Photoaging and Skin Protective Effects of Licania macrocarpa Cuatrec Methanol Extract
- PMID: 35631808
- PMCID: PMC9144732
- DOI: 10.3390/plants11101383
In Vitro Anti-Photoaging and Skin Protective Effects of Licania macrocarpa Cuatrec Methanol Extract
Abstract
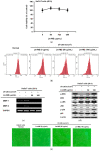
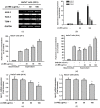
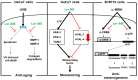
The Licania genus has been used in the treatment of dysentery, diabetes, inflammation, and diarrhea in South America. Of these plants, the strong anti-inflammatory activity of Licania macrocarpa Cuatrec (Chrysobalanaceae) has been reported previously. However, the beneficial activities of this plant on skin health have remained unclear. This study explores the protective activity of a methanol extract (50-100 μg/mL) in the aerial parts of L. macrocarpa Cuatrec (Lm-ME) and its mechanism, in terms of its moisturizing/hydration factors, skin wrinkles, UV radiation-induced cell damage, and radical generation (using RT/real-time PCR, carbazole assays, flowcytometry, DPPH/ABTS, and immunoblotting analysis). The anti-pigmentation role of Lm-ME was also tested by measuring levels of melanin, melanogenesis-related genes, and pigmentation-regulatory proteins. Lm-ME decreased UVB-irradiated death in HaCaT cells by suppressing apoptosis and inhibited matrix metalloproteinases 1/2 (MMP1/2) expression by enhancing the activity of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. It was confirmed that Lm-ME displayed strong antioxidative activity. Lm-ME upregulated the expression of hyaluronan synthases-2/3 (HAS-2/3) and transglutaminase-1 (TGM-1), as well as secreted levels of hyaluronic acid (HA) via p38 and JNK activation. This extract also significantly inhibited the production of hyaluronidase (Hyal)-1, -2, and -4. Lm-ME reduced the melanin expression of microphthalmia-associated transcription factor (MITF), tyrosinase, and tyrosinase-related protein-1/2 (TYRP-1/2) in α-melanocyte-stimulating hormone (α-MSH)-treated B16F10 cells via the reduction of cAMP response element-binding protein (CREB) and p38 activation. These results suggest that Lm-ME plays a role in skin protection through antioxidative, moisturizing, cytoprotective, and skin-lightening properties, and may become a new and promising cosmetic product beneficial for the skin.
Keywords: B16 melanoma cells; HaCaT cells; UVB exposure; melanogenesis; wrinkle formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Saba E., Kim S.H., Lee Y.Y., Park C.K., Oh J.W., Kim T.H., Kim H.K., Roh S.S., Rhee M.H. Korean red ginseng extract ameliorates melanogenesis in humans and induces antiphotoaging effects in ultraviolet B-irradiated hairless mice. J. Ginseng Res. 2020;44:496–505. doi: 10.1016/j.jgr.2019.05.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

