Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers
- PMID: 35632395
- PMCID: PMC9147589
- DOI: 10.3390/vaccines10050639
Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers
Abstract
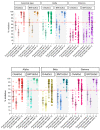
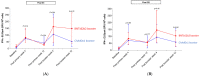
Inactivated SARS-CoV-2 vaccine (CoronaVac) is commonly used in national immunization programs. However, the immune response significantly declines within a few months. Our study assessed the immune response against SARS-CoV-2 after receiving booster shots of BNT162b2 or ChAdOx1 among health care workers who previously received CoronaVac as their primary immunization. Fifty-six participants who received ChAdOx1 and forty-two participants who received BNT162b2 were enrolled into this study, which evaluated immune responses, including anti-SARS-CoV-2 spike total antibodies (Elecsys®), surrogated viral neutralization test (sVNT) to ancestral strain (cPass™; GenScript), five variants of concern (Alpha, Beta, Gamma, Delta, and Omicron) (Luminex; multiplex sVNT) and the ELISpot with spike (S1 and S2) peptide pool against the ancestral SARS-CoV-2 strain. The samples were analyzed at baseline, 4, and 12 weeks after primary immunization, as well as 4 and 12 weeks after receiving the booster. This study showed a significant increase in anti-SARS-CoV-2 spike total antibodies, sVNT, and T-cell immune response after the booster, including against the Omicron variant. Immune responses rapidly decreased in the booster group at 12 weeks after booster but were still higher than post-primary vaccination. A fourth dose or a second booster should be recommended, particularly in health care workers.
Keywords: BNT162b2; COVID-19; ChAdOx1; CoronaVac; SARS-CoV-2; T-cell immune response; anti-SARS-CoV-2 spike total antibodies; booster; surrogate viral neutralizing antibody; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study.
Figures

References
-
- Jantarabenjakul W., Chantasrisawad N., Puthanakit T., Wacharapluesadee S., Hirankarn N., Ruenjaiman V., Paitoonpong L., Suwanpimolkul G., Torvorapanit P., Pradit R., et al. Short-term immune response after inactivated SARS-CoV-2 (CoronaVac(R), Sinovac) and ChAdOx1 nCoV-19 (Vaxzevria(R), Oxford-AstraZeneca) vaccinations in health care workers. Asian Pac. J. Allergy Immunol. 2021 doi: 10.12932/AP-250721-1197. - DOI - PubMed
-
- WHO. [(accessed on 5 March 2022)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
-
- Garcia-Beltran W.F., Denis K.J.S., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466. doi: 10.1016/j.cell.2021.12.033. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

