Effect of Heterologous Vaccination Strategy on Humoral Response against COVID-19 with CoronaVac plus BNT162b2: A Prospective Cohort Study
- PMID: 35632443
- PMCID: PMC9147346
- DOI: 10.3390/vaccines10050687
Effect of Heterologous Vaccination Strategy on Humoral Response against COVID-19 with CoronaVac plus BNT162b2: A Prospective Cohort Study
Abstract
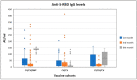
This study aimed to evaluate the mixed and homogeneous application of the inactivated SARS-CoV-2 vaccine CoronaVac (CV) and the mRNA vaccine BNT162b2 (BNT). This prospective cohort study included 235 health care workers who had received two prime shots with CoronaVac. They were divided into three cohorts after the third month: Cohort-I (CV/CV); Cohort-II (CV/CV/CV); and Cohort-III (CV/CV/BNT). Anti-S-RBD-IgG and total anti-spike/anti-nucleocapsid-IgG antibody concentrations were examined in vaccinated health workers at the 1st, 3rd, and 6th months following the second dose of the vaccination. The mean age of 235 health care workers who participated in the project was 39.51 ± 10.39 (min-max: 22-64). At the end of the 6th month, no antibodies were detected in 16.7% of Cohort-I participants, and anti-S-RDB IgG levels showed a decrease of 60% compared to the levels of the 3rd month. The antibody concentrations of the 6th month were found to have increased by an average of 5.13 times compared to the 3rd-month levels in Cohort-II and 20.4 times in Cohort-III. The heterologous vaccination strategy "CoronaVac and BNT162b2 regimen" is able to induce a stronger humoral immune response and it will help remove inequalities in the developing world where CoronaVac was the initial prime.
Keywords: BNT162 vaccine; COVID-19 vaccine booster shot; COVID-19 vaccines; heterologous vaccination; inactivated SARS-CoV2 vaccine; mixed vaccination; seroconversion; vaccination strategy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
-
- World Health Organization Draft Landscape of COVID-19 Candidate Vaccines. [(accessed on 5 February 2022)]. Available online: https://www.who.int/docs/default-source/a-future-for-children/novel-coro....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous