A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents
- PMID: 35632458
- PMCID: PMC9142924
- DOI: 10.3390/vaccines10050699
A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents
Abstract
Obesity can increase the severity of influenza infection. Data are limited regarding immune responses to influenza vaccination in obese children. We aimed to investigate the impact of obesity on quadrivalent influenza vaccine responses in children. Children with obesity (body mass index (BMI) ≥ 95th percentile for age and gender) and children without obesity (BMI < 95th percentile) were enrolled in the study. Blood samples were collected before, 1, and 6 months after influenza vaccination, to measure antibody responses by haemagglutination inhibition (HI) assay. Vaccine immunogenicity outcomes were compared between children with and without obesity. Forty-four children (mean age 13.3 ± 2.1 years, 18 males and 14 with obesity) completed the 6-month study. More than 90% of the participants with and without obesity had seroprotective antibody titres (HI ≥ 40) at both 1 and 6 months following vaccination for each of the four influenza strains (A/H3N2, A/H1N1, B/(Victoria) and B/(Yamagata)). Influenza-specific geometric mean titres at baseline, 1, and 6 months post-vaccination were similar between children with and without obesity for all influenza vaccine strains. Children with and without obesity have robust, sustained antibody responses over 6 months to the quadrivalent influenza vaccine.
Keywords: adolescents; children; influenza; obesity; vaccination.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M. Clarke, H.S. Marshall, P.C. Richmond, L.C. Giles and S.M. Mathew are investigators on clinical vaccine trials and their institutions receive research grants for investigator-led vaccine research/sponsored clinical vaccine trials from pharmaceutical industry companies including GSK, Pfizer, Sanofi, Merck. None of the authors received any personal payments from the Industry. Ian Barr has shares in an influenza vaccine producing company. The WHO CCRRI receives funds from Seqirus Limited and from the IFPMA for its influenza surveillance activities.
Figures

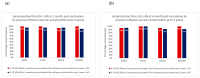
References
-
- World Health Organization 2021 Overweight and Obesity. [(accessed on 22 April 2022)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
-
- Australian Institute of Health and Welfare . Overweight and Obesity: An Interactive Insight. AIHW; Canberra, Australia: 2020. [(accessed on 17 September 2021)]. Available online: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesit....
-
- Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E., Kengne A.P., Hercberg S., Czernichow S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: A systematic review and meta-analysis. Obes. Rev. 2011;12:653–659. doi: 10.1111/j.1467-789X.2011.00864.x. - DOI - PubMed
-
- Moser J.A.S., Galindo-Fraga A., Ortiz-Hernández A.A., Gu W., Hunsberger S., Galán-Herrera J.F., Guerrero M.L., Ruiz-Palacios G.M., Beigel J.H., Magaña-Aquino M., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir. Viruses. 2019;13:3–9. doi: 10.1111/irv.12618. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

