New Onset of Eosinophilic Granulomatosis with Polyangiitis Following mRNA-Based COVID-19 Vaccine
- PMID: 35632472
- PMCID: PMC9144767
- DOI: 10.3390/vaccines10050716
New Onset of Eosinophilic Granulomatosis with Polyangiitis Following mRNA-Based COVID-19 Vaccine
Abstract
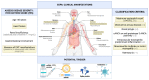
Anti-SARS-CoV-2 vaccines are safe and effective, also in individuals with allergic and immune-mediated diseases (IMDs). There are reports suggesting that vaccines may be able to trigger de-novo or exacerbate pre-existing IMDs in predisposed individuals. Eosinophilic granulomatosis with polyangiitis (EGPA) is a small-vessel vasculitis characterized by asthma, eosinophilia, and eosinophil-rich granulomatous inflammation in various tissues. We describe the case of a 63-year-old man who experienced cardiac, pulmonary, and neurological involvement one day after the administration of the booster dose of anti-SARS-CoV-2 vaccine (mRNA-1273). A diagnosis of EGPA was made and the patient was treated with high-dose steroids and cyclophosphamide, with a good clinical response. Interestingly, our patient had experienced a significant worsening of his pre-existing asthma six months earlier, just after the first two vaccine shots with the ChAdOx1 anti-SARS-CoV-2 vaccine. It is impossible to know whether our patient would have had developed EGPA following natural SARS-CoV-2 infection or at some point in his life regardless of infectious stimuli. Nevertheless, our report may suggest that caution should be paid during the administration of additional vaccine doses in individuals who experienced an increase in IMD severity that persisted over time following previous vaccine shots.
Keywords: ANCA-associated vasculitis; EGPA; anti-SARS-CoV-2 vaccine; mRNA vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Watad A., de Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., Haddad A., Elias M., Zisman D., Naffaa M.E., et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following MRNA/DNA SARS-CoV-2 Vaccination. Vaccines. 2021;9:435. doi: 10.3390/vaccines9050435. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

