High Prevalence of Non-Vaccinated Oncogenic Human Papillomavirus Genotypes in High-Grade Squamous Intraepithelial Lesions of the Cervix: Thought-Provoking Results of a Detailed HPV Genotype Analysis
- PMID: 35632504
- PMCID: PMC9146889
- DOI: 10.3390/vaccines10050748
High Prevalence of Non-Vaccinated Oncogenic Human Papillomavirus Genotypes in High-Grade Squamous Intraepithelial Lesions of the Cervix: Thought-Provoking Results of a Detailed HPV Genotype Analysis
Abstract
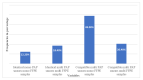
Identification of HPV infection is usually performed on cytological specimens, despite the often transient virus types. HPV profile analysis of pathologically confirmed lesions can also be performed on formalin-fixed paraffin-embedded (FFPE) cone samples and should be taken as standard during follow-up. We compared HPV profiles of cytological and FFPE specimens of women diagnosed with HSIL. Archived PAP smears and FFPE cones from 49 patients were processed. For genotyping, the HPV Direct Flow CHIP test was used. All samples were positive. HPV profile agreement of the two sample types was 84.16-100%. Mono-infections occurred in 12.24% and 61.22% in PAP smears and FFPE specimens, respectively; while multi-infections were detected in 87.76% and 38.78%, respectively. The most abundant genotypes were HPVs 16, 31, and 51/33. Of all infections, 56.25% and 64.93% were caused by nonavalent vaccinated type (VT) HPVs; while 50.69% and 38.96% belonged to non-nonavalent VT HPVs, in PAP smears and FFPE specimens, respectively. Our results confirmed the importance of HPV genotyping of FFPE cone samples. We also confirmed a remarkable presence of non-vaccinated HPV types in HSIL cases indicating the importance of vaccine development.
Keywords: FFPE cone sample; HPV Direct Flow CHIP; HPV genotype profile; HSIL; PAP smear; nonavalent HPV vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The prevelance of human papillomavirus (HPV) genotypes detected by PCR in women with normal and abnormal cervico-vaginal cytology.Ginekol Pol. 2018;89(2):62-67. doi: 10.5603/GP.a2018.0011. Ginekol Pol. 2018. PMID: 29512809
-
Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 korean women determined by DNA chip.Cancer Epidemiol Biomarkers Prev. 2004 Dec;13(12):2153-6. Cancer Epidemiol Biomarkers Prev. 2004. PMID: 15598774
-
Evaluation of the detection of human papillomavirus genotypes in cervical specimens by hybrid capture as screening for precancerous lesions in HIV-positive women.J Med Virol. 1998 Oct;56(2):133-7. doi: 10.1002/(sici)1096-9071(199810)56:2<133::aid-jmv6>3.0.co;2-9. J Med Virol. 1998. PMID: 9746069
-
Genotype distribution of human papillomaviruses in Japanese women with abnormal cervical cytology.Open Virol J. 2012;6:277-83. doi: 10.2174/1874357901206010277. Epub 2012 Dec 28. Open Virol J. 2012. PMID: 23341864 Free PMC article.
-
The nonavalent vaccine: a review of high-risk HPVs and a plea to the CDC.Am J Stem Cells. 2019 Dec 15;8(3):52-64. eCollection 2019. Am J Stem Cells. 2019. PMID: 31976155 Free PMC article. Review.
Cited by
-
High Prevalence of HPV 51 in an Unvaccinated Population and Implications for HPV Vaccines.Vaccines (Basel). 2022 Oct 20;10(10):1754. doi: 10.3390/vaccines10101754. Vaccines (Basel). 2022. PMID: 36298619 Free PMC article.
-
Infectious Agents Induce Wnt/β-Catenin Pathway Deregulation in Primary Liver Cancers.Microorganisms. 2023 Jun 22;11(7):1632. doi: 10.3390/microorganisms11071632. Microorganisms. 2023. PMID: 37512809 Free PMC article. Review.
-
Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study.Vaccines (Basel). 2025 Jun 13;13(6):640. doi: 10.3390/vaccines13060640. Vaccines (Basel). 2025. PMID: 40573971 Free PMC article.
References
-
- Papillomavirus Episteme: A Comprehensive Papillomaviridae Database and Analysis Resource. [(accessed on 24 February 2022)];2012 Available online: https://pave.niaid.nih.gov/#home.
-
- WHO. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 90 Human Papillomaviruses; IARC; Lyon, France: 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

