Vaccination against Bacterial Infections: Challenges, Progress, and New Approaches with a Focus on Intracellular Bacteria
- PMID: 35632507
- PMCID: PMC9144739
- DOI: 10.3390/vaccines10050751
Vaccination against Bacterial Infections: Challenges, Progress, and New Approaches with a Focus on Intracellular Bacteria
Abstract
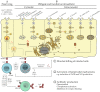
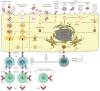
Many bacterial infections are major health problems worldwide, and treatment of many of these infectious diseases is becoming increasingly difficult due to the development of antibiotic resistance, which is a major threat. Prophylactic vaccines against these bacterial pathogens are urgently needed. This is also true for bacterial infections that are still neglected, even though they affect a large part of the world's population, especially under poor hygienic conditions. One example is typhus, a life-threatening disease also known as "war plague" caused by Rickettsia prowazekii, which could potentially come back in a war situation such as the one in Ukraine. However, vaccination against bacterial infections is a challenge. In general, bacteria are much more complex organisms than viruses and as such are more difficult targets. Unlike comparatively simple viruses, bacteria possess a variety of antigens whose immunogenic potential is often unknown, and it is unclear which antigen can elicit a protective and long-lasting immune response. Several vaccines against extracellular bacteria have been developed in the past and are still used successfully today, e.g., vaccines against tetanus, pertussis, and diphtheria. However, while induction of antibody production is usually sufficient for protection against extracellular bacteria, vaccination against intracellular bacteria is much more difficult because effective defense against these pathogens requires T cell-mediated responses, particularly the activation of cytotoxic CD8+ T cells. These responses are usually not efficiently elicited by immunization with non-living whole cell antigens or subunit vaccines, so that other antigen delivery strategies are required. This review provides an overview of existing antibacterial vaccines and novel approaches to vaccination with a focus on immunization against intracellular bacteria.
Keywords: antigens; extracellular and intracellular bacteria; immunity; vaccine.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections.Life Sci. 2023 Feb 1;314:121332. doi: 10.1016/j.lfs.2022.121332. Epub 2022 Dec 28. Life Sci. 2023. PMID: 36584914 Review.
-
Vaccine Design and Vaccination Strategies against Rickettsiae.Vaccines (Basel). 2021 Aug 12;9(8):896. doi: 10.3390/vaccines9080896. Vaccines (Basel). 2021. PMID: 34452021 Free PMC article. Review.
-
Feasible improvements in vaccines in the Expanded Programme on Immunization.Rev Infect Dis. 1989 May-Jun;11 Suppl 3:S530-7. doi: 10.1093/clinids/11.supplement_3.s530. Rev Infect Dis. 1989. PMID: 2669097 Review.
-
Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens.Expert Rev Vaccines. 2004 Feb;3(1):23-34. doi: 10.1586/14760584.3.1.23. Expert Rev Vaccines. 2004. PMID: 14761241 Review.
-
Vaccine development: obligate intracellular bacteria new tools, old pathogens: the current state of vaccines against obligate intracellular bacteria.Front Cell Infect Microbiol. 2024 Mar 19;14:1282183. doi: 10.3389/fcimb.2024.1282183. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38567021 Free PMC article. Review.
Cited by
-
Mining gene expression data for rational identification of novel drug targets and vaccine candidates against the cattle tick, Rhipicephalus microplus.Exp Appl Acarol. 2023 Oct;91(2):291-317. doi: 10.1007/s10493-023-00838-8. Epub 2023 Sep 27. Exp Appl Acarol. 2023. PMID: 37755526 Free PMC article.
-
Nanobiotics against antimicrobial resistance: harnessing the power of nanoscale materials and technologies.J Nanobiotechnology. 2022 Aug 12;20(1):375. doi: 10.1186/s12951-022-01573-9. J Nanobiotechnology. 2022. PMID: 35953826 Free PMC article. Review.
-
Subtractive proteomics and reverse-vaccinology approaches for novel drug targets and designing a chimeric vaccine against Ruminococcus gnavus strain RJX1120.Front Immunol. 2025 Apr 14;16:1555741. doi: 10.3389/fimmu.2025.1555741. eCollection 2025. Front Immunol. 2025. PMID: 40297578 Free PMC article.
-
Current challenges and improvements in assessing the immunogenicity of bacterial vaccines.Front Microbiol. 2024 Jul 9;15:1404637. doi: 10.3389/fmicb.2024.1404637. eCollection 2024. Front Microbiol. 2024. PMID: 39044946 Free PMC article. Review.
-
mRNA vaccines for infectious diseases - advances, challenges and opportunities.Nat Rev Drug Discov. 2024 Nov;23(11):838-861. doi: 10.1038/s41573-024-01042-y. Epub 2024 Oct 4. Nat Rev Drug Discov. 2024. PMID: 39367276 Review.
References
-
- Chong A., Wehrly T.D., Nair V., Fischer E.R., Barker J.R., Klose K.E., Celli J. The Early Phagosomal Stage of Francisella tularensis Determines Optimal Phagosomal Escape and Francisella Pathogenicity Island Protein Expression. Infect. Immun. 2008;76:5488–5499. doi: 10.1128/IAI.00682-08. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

