Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic
- PMID: 35632512
- PMCID: PMC9146933
- DOI: 10.3390/vaccines10050755
Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic
Abstract
The incidence of COVID-19 breakthrough infections-an infection that occurs after you have been vaccinated-has increased in frequency since the Delta and now Omicron variants of the SARS-CoV-2 coronavirus have become the dominant strains transmitted in the United States (US). Evidence suggests that individuals with breakthrough infections, though rare and expected, may readily transmit COVID-19 to unvaccinated populations, posing a continuing threat to the unvaccinated. Here, we examine factors contributing to breakthrough infections including a poor immune response to the vaccines due to the fact of advanced age and underlying comorbidities, the natural waning of immune protection from the vaccines over time, and viral variants that escape existing immune protection from the vaccines. The rise in breakthrough infections in the US and how they contribute to new infections, specifically among the unvaccinated and individuals with compromised immune systems, will create the need for additional booster vaccinations or development of modified vaccines that directly target current variants circulating among the general population. The need to expedite vaccination among the more than 49.8 million unvaccinated eligible people in the US is critical.
Keywords: COVID-19; Delta variant; Omicron variant; SARS-CoV-2; breakthrough infections; coronavirus; vaccine.
Conflict of interest statement
The authors declare no existence of competing interest. The funders did not participate in the design, preparation, or data analysis of the study or the decision to publish the manuscript.
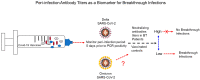
Figures




References
-
- Salacup G., Lo K.B., Gul F., Peterson E., De Joy R., Bhargav R., Pelayo J., Albano J., Azmaiparashvili Z., Patarroyo-Aponte G., et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. J. Med. Virol. 2021;93:416–423. doi: 10.1002/jmv.26252. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

