Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication
- PMID: 35632611
- PMCID: PMC9144315
- DOI: 10.3390/v14050869
Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication
Abstract
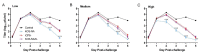
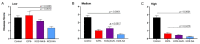
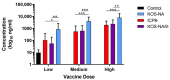
We previously isolated an HSV-1 mutant, KOS-NA, that contains two non-synonymous mutations in UL39. One of the mutations, resulting in an R950H amino acid substitution in ICP6, renders KOS-NA severely neuro-attenuated and significantly reduces HSV-1 latency. Vaccination of mice with KOS-NA prior to corneal challenge provides significant protection against HSV-1-mediated eye diseases even at a very low immunizing dose, indicating its utility as a vaccine scaffold. Because KOS-NA contains a neuro-attenuating mutation in a single gene, we sought to improve its safety by deleting a portion of the UL29 gene whose protein product, ICP8, is essential for viral DNA replication. Whereas KOS-NA reduced replication of HSV-1 challenge virus in the corneal epithelium and protected mice against blepharitis and keratitis induced by the challenge virus, KOS-NA/8- and an ICP8- virus were significantly less efficacious except at higher doses. Our results suggest that the capacity to replicate, even at significantly reduced levels compared with wild-type HSV-1, may be an important feature of an effective vaccine. Means to improve safety of attenuated viruses as vaccines without compromising efficacy should be sought.
Keywords: corneal infection; herpes simplex virus 1; replication-defective; vaccine.
Conflict of interest statement
The authors hold a patent (U.S. patent 9616119) on the use of KOS-NA as a vaccine against HSV-1. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
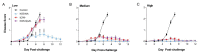


References
-
- Roizman R., Knipe D.M., Whitley R.J. Herpes simplex viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott Williams & Wilkins; New York, NY, USA: 2007. pp. 2501–2601.
-
- Barron B.A., Gee L., Hauck W.W., Kurinij N., Dawson C.R., Jones D.B., Wilhelmus K.R., Kaufman H.E., Sugar J., Hyndiuk R.A., et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–1882. doi: 10.1016/S0161-6420(13)31155-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

