Generated Randomly and Selected Functionally? The Nature of Enterovirus Recombination
- PMID: 35632658
- PMCID: PMC9144335
- DOI: 10.3390/v14050916
Generated Randomly and Selected Functionally? The Nature of Enterovirus Recombination
Abstract
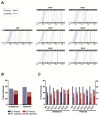
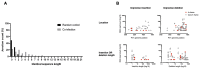
Genetic recombination in RNA viruses is an important evolutionary mechanism. It contributes to population diversity, host/tissue adaptation, and compromises vaccine efficacy. Both the molecular mechanism and initial products of recombination are relatively poorly understood. We used an established poliovirus-based in vitro recombination assay to investigate the roles of sequence identity and RNA structure, implicated or inferred from an analysis of circulating recombinant viruses, in the process. In addition, we used next-generation sequencing to investigate the early products of recombination after cellular coinfection with different poliovirus serotypes. In independent studies, we find no evidence for a role for RNA identity or structure in determining recombination junctions location. Instead, genome function and fitness are of greater importance in determining the identity of recombinant progeny. These studies provide further insights into this important evolutionary mechanism and emphasize the critical nature of the selection process on a mixed virus population.
Keywords: next-generation sequencing; positive-sense RNA viruses; recombination; viral evolution.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



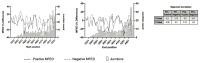
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

