Genomic and In Vitro Phenotypic Comparisons of Epidemic and Non-Epidemic Getah Virus Strains
- PMID: 35632684
- PMCID: PMC9145621
- DOI: 10.3390/v14050942
Genomic and In Vitro Phenotypic Comparisons of Epidemic and Non-Epidemic Getah Virus Strains
Abstract
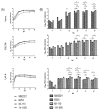
Getah virus is an emerging mosquito-borne animal pathogen. Four phylogenetic groups of GETV, Group I (GI), GII, GIII and GIV, were identified. However, only the GETV GIII was associated with disease epidemics suggesting possible virulence difference in this virus group. Here, we compared the genetic and in vitro phenotypic characteristics between the epidemic and non-epidemic GETV. Our complete coding genome sequence analyses revealed several amino acid substitutions unique to the GETV GIII and GIV groups, which were found mainly in the hypervariable domain of nsP3 and E2 proteins. Replication kinetics of the epidemic (GIII MI-110 and GIII 14-I-605) and non-epidemic GETV strains (prototype GI MM2021 and GIV B254) were compared in mammalian Vero cells and mosquito C6/36 and U4.4 cells. In all cells used, both epidemic GETV GIII MI-110 and GIII 14-I-605 strains showed replication rates and mean maximum titers at least 2.7-fold and 2.3-fold higher than those of GIV B254, respectively (Bonferroni posttest, p < 0.01). In Vero cells, the epidemic GETV strains caused more pronounced cytopathic effects in comparison to the GIV B254. Our findings suggest that higher virus replication competency that produces higher virus titers during infection may be the main determinant of virulence and epidemic potential of GETV.
Keywords: Getah virus; alphavirus; arbovirus; emerging; infectious diseases; tropical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Weaver S.C.F.T., Huang H.V., Kinney R.M., Rice C.M., Roehrig J.T., Shope R.E., Strauss E.G. Togaviridae. Virus Taxon. 2005;8:10.
-
- BERGE T. International Catalogue of Arbovirus, Including Certain Other Viruses of Vertebrates. CDC Public Health Service, Department of Health Education and Welfare; Atlanta, GA, USA: 1975.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

