Seroprevalence of SARS-CoV-2 IgG Antibodies and Factors Associated with SARS-CoV-2 IgG Neutralizing Activity among Primary Health Care Workers 6 Months after Vaccination Rollout in France
- PMID: 35632699
- PMCID: PMC9148144
- DOI: 10.3390/v14050957
Seroprevalence of SARS-CoV-2 IgG Antibodies and Factors Associated with SARS-CoV-2 IgG Neutralizing Activity among Primary Health Care Workers 6 Months after Vaccination Rollout in France
Abstract
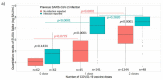
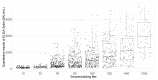
We aimed to investigate the immunoglobulin G response and neutralizing activity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) among primary health care workers (PHCW) in France and assess the association between the neutralizing activity and several factors, including the coronavirus disease 2019 (COVID-19) vaccination scheme. A cross-sectional survey was conducted between 10 May 2021 and 31 August 2021. Participants underwent capillary blood sampling and completed a questionnaire. Sera were tested for the presence of antibodies against the nucleocapsid (N) protein and the S-1 portion of the spike (S) protein and neutralizing antibodies. In total, 1612 PHCW were included. The overall seroprevalences were: 23.6% (95% confidence interval (CI) 21.6-25.7%) for antibodies against the N protein, 94.7% (93.6-95.7%) for antibodies against the S protein, and 81.3% (79.4-83.2%) for neutralizing antibodies. Multivariate regression analyses showed that detection of neutralizing antibodies was significantly more likely in PHCW with previous SARS-CoV-2 infection than in those with no such history among the unvaccinated (odds ratio (OR) 16.57, 95% CI 5.96-59.36) and those vaccinated with one vaccine dose (OR 41.66, 95% CI 16.05-120.78). Among PHCW vaccinated with two vaccine doses, the detection of neutralizing antibodies was not significantly associated with previous SARS-CoV-2 infection (OR 1.31, 95% CI 0.86-2.07), but was more likely in those that received their second vaccine dose within the three months before study entry than in those vaccinated more than three months earlier (OR 5.28, 95% CI 3.51-8.23). This study highlights that previous SARS-CoV-2 infection and the time since vaccination should be considered when planning booster doses and the design of COVID-19 vaccine strategies.
Keywords: SARS-CoV-2 antibodies; health care workers; neutralizing antibodies; primary care; seroprevalence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
-
- Haute Autorité de Santé Stratégie de Vaccination Contre le SARS-CoV-2—Vaccination des Personnes Ayant un Antécédent de COVID-19. 2021. [(accessed on 2 March 2022)]. Available online: https://www.has-sante.fr/jcms/p_3237355/fr/strategie-de-vaccination-cont....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

