A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus
- PMID: 35632742
- PMCID: PMC9147793
- DOI: 10.3390/v14051000
A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus
Abstract
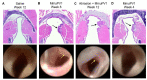
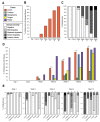
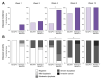
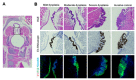
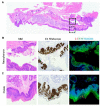
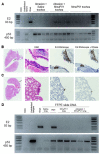
Recurrent respiratory papillomatosis (RRP), caused by laryngeal infection with low-risk human papillomaviruses, has devastating effects on vocal communication and quality of life. Factors in RRP onset, other than viral presence in the airway, are poorly understood. RRP research has been stalled by limited preclinical models. The only known papillomavirus able to infect laboratory mice, Mus musculus papillomavirus (MmuPV1), induces disease in a variety of tissues. We hypothesized that MmuPV1 could infect the larynx as a foundation for a preclinical model of RRP. We further hypothesized that epithelial injury would enhance the ability of MmuPV1 to cause laryngeal disease, because injury is a potential factor in RRP and promotes MmuPV1 infection in other tissues. In this report, we infected larynges of NOD scid gamma mice with MmuPV1 with and without vocal fold abrasion and measured infection and disease pathogenesis over 12 weeks. Laryngeal disease incidence and severity increased earlier in mice that underwent injury in addition to infection. However, laryngeal disease emerged in all infected mice by week 12, with or without injury. Secondary laryngeal infections and disease arose in nude mice after MmuPV1 skin infections, confirming that experimentally induced injury is dispensable for laryngeal MmuPV1 infection and disease in immunocompromised mice. Unlike RRP, lesions were relatively flat dysplasias and they could progress to cancer. Similar to RRP, MmuPV1 transcript was detected in all laryngeal disease and in clinically normal larynges. MmuPV1 capsid protein was largely absent from the larynx, but productive infection arose in a case of squamous metaplasia at the level of the cricoid cartilage. Similar to RRP, disease spread beyond the larynx to the trachea and bronchi. This first report of laryngeal MmuPV1 infection provides a foundation for a preclinical model of RRP.
Keywords: MmuPV1; RRP; laryngoscopy; larynx; mouse; papillomavirus; recurrent respiratory papillomatosis; vocal folds.
Conflict of interest statement
Sales of the mouse anesthesia nose cone are licensed through the Wisconsin Alumni Research Foundation. The inventors (including A.B. and P.F.L.) will receive a portion of the proceeds. R.E.K., J.R., E.T.W-S., R.H. and S.L.T. declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- F31 DC018184/DC/NIDCD NIH HHS/United States
- P50 DE026787/NH/NIH HHS/United States
- T32 DC009401/NH/NIH HHS/United States
- F31 DC018184/NH/NIH HHS/United States
- R01 DC004336/DC/NIDCD NIH HHS/United States
- P01 CA022443/NH/NIH HHS/United States
- T32 CA090217/NH/NIH HHS/United States
- R01 DC004336/NH/NIH HHS/United States
- R35 CA210807/CA/NCI NIH HHS/United States
- P01 CA022443/CA/NCI NIH HHS/United States
- P30 CA014520/NH/NIH HHS/United States
- T32 DC009401/DC/NIDCD NIH HHS/United States
- P50 DE026787/DE/NIDCR NIH HHS/United States
- R35 CA210807/NH/NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- T32 CA090217/CA/NCI NIH HHS/United States
- P50 CA278595/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

