PAI-1 production by reactive astrocytes drives tissue dysfibrinolysis in multiple sclerosis models
- PMID: 35633384
- PMCID: PMC11072877
- DOI: 10.1007/s00018-022-04340-z
PAI-1 production by reactive astrocytes drives tissue dysfibrinolysis in multiple sclerosis models
Abstract
Background: In multiple sclerosis (MS), disturbance of the plasminogen activation system (PAS) and blood brain barrier (BBB) disruption are physiopathological processes that might lead to an abnormal fibrin(ogen) extravasation into the parenchyma. Fibrin(ogen) deposits, usually degraded by the PAS, promote an autoimmune response and subsequent demyelination. However, the PAS disruption is not well understood and not fully characterized in this disorder.
Methods: Here, we characterized the expression of PAS actors during different stages of two mouse models of MS (experimental autoimmune encephalomyelitis-EAE), in the central nervous system (CNS) by quantitative RT-PCR, immunohistofluorescence and fluorescent in situ hybridization (FISH). Thanks to constitutive PAI-1 knockout mice (PAI-1 KO) and an immunotherapy using a blocking PAI-1 antibody, we evaluated the role of PAI-1 in EAE models and its impact on physiopathological processes such as fibrin(ogen) deposits, lymphocyte infiltration and demyelination.
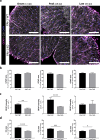
Results: We report a striking overexpression of PAI-1 in reactive astrocytes during symptomatic phases, in two EAE mouse models of MS. This increase is concomitant with lymphocyte infiltration and fibrin(ogen) deposits in CNS parenchyma. By genetic invalidation of PAI-1 in mice and immunotherapy using a blocking PAI-1 antibody, we demonstrate that abolition of PAI-1 reduces the severity of EAE and occurrence of relapses in two EAE models. These benefits are correlated with a decrease in fibrin(ogen) deposits, infiltration of T4 lymphocytes, reactive astrogliosis, demyelination and axonal damage.
Conclusion: These results demonstrate that a deleterious overexpression of PAI-1 by reactive astrocytes leads to intra-parenchymal dysfibrinolysis in MS models and anti-PAI-1 strategies could be a new therapeutic perspective for MS.
Keywords: Astrocytes; EAE; Intraparenchymal fibrinolysis; PAI-1.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

