Identification of oxazolo[4,5-g]quinazolin-2(1H)-one Derivatives as EGFR Inhibitors for Cancer Prevention
- PMID: 35633554
- PMCID: PMC9587877
- DOI: 10.31557/APJCP.2022.23.5.1687
Identification of oxazolo[4,5-g]quinazolin-2(1H)-one Derivatives as EGFR Inhibitors for Cancer Prevention
Abstract
Objective: Abnormal expression of EGFR (epidermal growth factor receptor) results in different types of human tumors. Quinazoline-containing derivative signify an attractive platform for EGFR inhibitors. The present study aims to discover the potential binders of a group of compounds belonging to oxazolo[4,5-g]quinazoline-2(1H)-one derivative as EGFR inhibitors.
Methods: We apply multiple computational procedures, including pharmacophore-based virtual screening methods, to perform a preliminary screening against EGFR over compounds belonging to oxazolo[4,5-g]quinazoline-2(1H)-one derivative. Then, we carried a fine screening by molecular dynamics simulations, followed by free energy calculations.
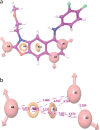
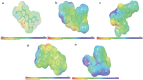
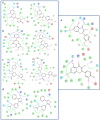
Results: The best pharmacophore model created has five characteristics. Three hydrogen bonds acceptors (A) and two aromatic rings (R) make up AAARR (a sequential representation of chemical features of ligands). We have performed pharmacophore-based screening with different databases like Asinex, Chembridge, Lifechemicals, Maybridge, Specs, and Zinc. Top-scoring 30 molecules were considered for performing induced fit docking. Molecular Dynamics Simulations executed for the top five ligands confirmed that throughout the simulation, the protein-ligand complexes remained stable.
Conclusion: Thus, the results obtained from this research provide insights for the development of oxazolo[4,5-g]quinazoline-2(1H)-one derivative as potent EGFR inhibitors.
Keywords: Tyrosine kinase; cancer biology; drug discovery; inhibitor discovery; pharmacophore-based virtual screening.
Figures


References
-
- Abeer I, Khan S, Hasan MJ, Hussain M. EGFR and HER2neu Expression in Gall Bladder Carcinoma and Its Association with Clinicopathological Parameters - An Institutional Experience from North India. Asian Pac J Cancer Biol. 2021;6:57–65.
-
- Becke AD. Density-functional thermochemistry II The role of exact exchange. J Chem Phys. 1993;98:5648.
-
- Bochevarov AD, Harder E, Hughes TF, et al. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int J Quantum Chem. 2013;113:2110–42.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

