Biocontrol and Action Mechanism of Bacillus subtilis Lipopeptides' Fengycins Against Alternaria solani in Potato as Assessed by a Transcriptome Analysis
- PMID: 35633712
- PMCID: PMC9130778
- DOI: 10.3389/fmicb.2022.861113
Biocontrol and Action Mechanism of Bacillus subtilis Lipopeptides' Fengycins Against Alternaria solani in Potato as Assessed by a Transcriptome Analysis
Abstract
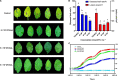
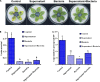
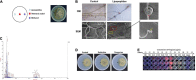
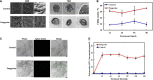
Alternaria solani is an airborne fungus and the primary causal agent of potato early blight worldwide. No available fungicides that are both effective and environmentally friendly are usable to control this fungus. Therefore, biological control is a potential approach for its suppression. In this study, Bacillus subtilis strain ZD01's fermentation broth strongly reduced A. solani pathogenicity under greenhouse conditions. The effects of strain ZD01's secondary metabolites on A. solani were investigated. The exposure of A. solani hyphae to the supernatant resulted in swelling and swollen sacs, and the ZD01 supernatant reduced A. solani conidial germination significantly. Matrix-assisted laser desorption/ionization time of flight mass spectrometry and pure product tests revealed that fengycins were the main antifungal lipopeptide substances. To elucidate the molecular mechanism of the fengycins' biological control, RNA sequencing analyses were performed. A transcriptome analysis revealed that 304 and 522 genes in A. solani were differentially expressed after 2-h and 6-h fengycin treatments, respectively. These genes were respectively mapped to 53 and 57 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. In addition, the most enriched KEGG pathway analysis indicated that the inhibitory mechanisms of fengycins against A. solani regulated the expression of genes related to cell wall, cell membrane, transport, energy process, protein synthesis and genetic information. In particular, cell wall and cell membrane metabolism were the main processes affected by fengycin stress. Scanning and transmission electron microscope results revealed hyphal enlargement and a wide range of abnormalities in A. solani cells after exposure to fengycins. Furthermore, fengycins induced chitin synthesis in treated cells, and also caused the capture of cellular fluorescent green labeling and the release of adenosine triphosphate (ATP) from outer membranes of A. solani cells, which may enhance the fengycins ability to alter cell membrane permeability. Thus, this study increases the transcriptome data resources available and supplies a molecular framework for B. subtilis ZD01 inhibition of A. solani HWC-168 through various mechanisms, especially damaging A. solani cell walls and membranes. The transcriptomic insights may lead to an effective control strategy for potato early blight.
Keywords: Alternaria solani; Bacillus subtilis; cell wall and membrane; conidia; fengycins; transcriptome.
Copyright © 2022 Zhang, Qiang, Zhou, Pan, Yu, Yuan, Cheng, Wang, Zhao, Zhu and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Davidson R. D., Houser A. J., Haslar R. (2015). Control of early blight in the San Luis Valley, Colorado. Am. J. Potato Res. 93 43–49. 10.1007/s12230-015-9482-4 - DOI
-
- De Lira Mota K. S., De Oliveira Pereira F., De Oliveira W. A., Lima I. O., De Oliveira Lima E. (2012). Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: interaction with ergosterol. Molecules 17 14418–14433. 10.3390/molecules171214418 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

