Photocatalytic Late-Stage Functionalization of Sulfonamides via Sulfonyl Radical Intermediates
- PMID: 35633900
- PMCID: PMC9127806
- DOI: 10.1021/acscatal.2c01442
Photocatalytic Late-Stage Functionalization of Sulfonamides via Sulfonyl Radical Intermediates
Abstract
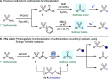
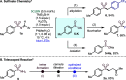
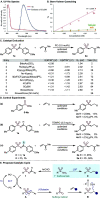
A plethora of drug molecules and agrochemicals contain the sulfonamide functional group. However, sulfonamides are seldom viewed as synthetically useful functional groups. To confront this limitation, a late-stage functionalization strategy is described, which allows sulfonamides to be converted to pivotal sulfonyl radical intermediates. This methodology exploits a metal-free photocatalytic approach to access radical chemistry, which is harnessed by combining pharmaceutically relevant sulfonamides with an assortment of alkene fragments. Additionally, the sulfinate anion can be readily obtained, further broadening the options for sulfonamide functionalization. Mechanistic studies suggest that energy-transfer catalysis (EnT) is in operation.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Photoredox Catalysis-Enabled Sulfination of Alcohols and Bromides.J Am Chem Soc. 2023 Sep 27;145(38):20767-20774. doi: 10.1021/jacs.3c08216. Epub 2023 Sep 18. J Am Chem Soc. 2023. PMID: 37721547 Free PMC article.
-
Primary Sulfonamide Functionalization via Sulfonyl Pyrroles: Seeing the N-Ts Bond in a Different Light.Chemistry. 2021 Nov 5;27(62):15387-15391. doi: 10.1002/chem.202102748. Epub 2021 Sep 30. Chemistry. 2021. PMID: 34409663
-
EnT-Mediated Amino-Sulfonylation of Alkenes with Bifunctional Sulfonamides: Access to β-Amino Sulfone Derivatives.Chemistry. 2023 Aug 1;29(43):e202301392. doi: 10.1002/chem.202301392. Epub 2023 Jun 28. Chemistry. 2023. PMID: 37218305
-
Photocatalytic Late-Stage C-H Functionalization.Chem Rev. 2023 Apr 26;123(8):4237-4352. doi: 10.1021/acs.chemrev.2c00478. Epub 2023 Jan 24. Chem Rev. 2023. PMID: 36692361 Review.
-
Decoding Directing Groups and Their Pivotal Role in C-H Activation.Chemistry. 2021 Sep 1;27(49):12453-12508. doi: 10.1002/chem.202101004. Epub 2021 Jul 13. Chemistry. 2021. PMID: 34038596 Review.
Cited by
-
Metal-free synthesis of γ-ketosulfones through Brønsted acid-promoted conjugate addition of sulfinamides.RSC Adv. 2024 Feb 5;14(7):4623-4631. doi: 10.1039/d3ra08675e. eCollection 2024 Jan 31. RSC Adv. 2024. PMID: 38318627 Free PMC article.
-
Advances in the construction of diverse SuFEx linkers.Natl Sci Rev. 2023 Apr 29;10(6):nwad123. doi: 10.1093/nsr/nwad123. eCollection 2023 Jun. Natl Sci Rev. 2023. PMID: 37441224 Free PMC article. Review.
-
Formal [4 + 2] combined ionic and radical approach of vinylogous enaminonitriles to access highly substituted sulfonyl pyridazines.Commun Chem. 2024 Nov 30;7(1):281. doi: 10.1038/s42004-024-01368-z. Commun Chem. 2024. PMID: 39616260 Free PMC article.
-
Energy-transfer-enabled photocatalytic transformations of aryl thianthrenium salts.Nat Commun. 2024 Nov 8;15(1):9693. doi: 10.1038/s41467-024-54079-3. Nat Commun. 2024. PMID: 39516492 Free PMC article.
-
Preassembly-Controlled Radical Recombination at Bismuth: Decarboxylative C─N Coupling with Sulfonamides.Chemistry. 2025 May 19;31(28):e202500396. doi: 10.1002/chem.202500396. Epub 2025 Apr 21. Chemistry. 2025. PMID: 40192563 Free PMC article.
References
-
- Lesch J. E.The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine; Oxford University Press: Oxford, 2007.
-
- McGrath N. A.; Brichacek M.; Njardarson J. T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Ed. 2010, 87, 1348–1349. 10.1021/ed1003806. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
