Recent Advances in Solid Tumor CAR-T Cell Therapy: Driving Tumor Cells From Hero to Zero?
- PMID: 35634281
- PMCID: PMC9130586
- DOI: 10.3389/fimmu.2022.795164
Recent Advances in Solid Tumor CAR-T Cell Therapy: Driving Tumor Cells From Hero to Zero?
Abstract
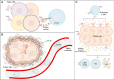
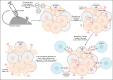
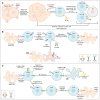
Chimeric antigen receptor T-cells (CAR-Ts) are known as revolutionary living drugs that have turned the tables of conventional cancer treatments in certain hematologic malignancies such as B-cell acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL) by achieving US Food and Drug Administration (FDA) approval based on their successful clinical outcomes. However, this type of therapy has not seen the light of victory in the fight against solid tumors because of various restricting caveats including heterogeneous tumor antigen expression and the immunosuppressive tumor microenvironments (TME) that negatively affect the tumor-site accessibility, infiltration, stimulation, activation, and persistence of CAR-Ts. In this review, we explore strategic twists including boosting vaccines and designing implementations that can support CAR-T expansion, proliferation, and tumoricidal capacity. We also step further by underscoring novel strategies for triggering endogenous antitumor responses and overcoming the limitation of poor CAR-T tumor-tissue infiltration and the lack of definitive tumor-specific antigens. Ultimately, we highlight how these approaches can address the mentioned arduous hurdles.
Keywords: chimeric antigen receptor; immunotherapy; infiltration; solid tumors; tumor microenvironment; vaccines.
Copyright © 2022 Safarzadeh Kozani, Safarzadeh Kozani, Ahmadi Najafabadi, Yousefi, Mirarefin and Rahbarizadeh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

