Humoral Immune Response Diversity to Different COVID-19 Vaccines: Implications for the "Green Pass" Policy
- PMID: 35634315
- PMCID: PMC9130843
- DOI: 10.3389/fimmu.2022.833085
Humoral Immune Response Diversity to Different COVID-19 Vaccines: Implications for the "Green Pass" Policy
Abstract
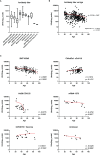
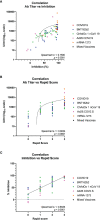
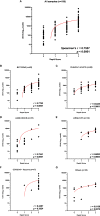
In the COVID-19 pandemic year 2021, several countries have implemented a vaccine certificate policy, the "Green Pass Policy" (GPP), to reduce virus spread and to allow safe relaxation of COVID-19 restrictions and reopening of social and economic activities. The rationale for the GPP is based on the assumption that vaccinated people should maintain a certain degree of immunity to SARS-CoV-2. Here we describe and compare, for the first time, the humoral immune response to mRNA-1273, BNT162b2, Ad26.COV2.S, and ChAdOx1 nCoV-19 vaccines in terms of antibody titer elicited, neutralizing activity, and epitope reactogenicity among 369 individuals aged 19 to 94 years. In parallel, we also considered the use of a rapid test for the determination of neutralizing antibodies as a tool to guide policymakers in defining booster vaccination strategies and eligibility for Green Pass. Our analysis demonstrates that the titer of antibodies directed towards the receptor-binding domain (RBD) of SARS-CoV-2 Spike is significantly associated with age and vaccine type. Moreover, natural COVID-19 infection combined with vaccination results, on average, in higher antibody titer and higher neutralizing activity as compared to fully vaccinated individuals without prior COVID-19. We also found that levels of anti-Spike RBD antibodies are not always strictly associated with the extent of inhibition of RBD-ACE2 binding, as we could observe different neutralizing activities in sera with similar anti-RBD concentrations. Finally, we evaluated the reactivity to four synthetic peptides derived from Spike protein on a randomly selected serum sample and observed that similar to SARS-CoV-2 infection, vaccination elicits a heterogeneous antibody response with qualitative individual features. On the basis of our results, the use of rapid devices to detect the presence of neutralizing antibodies, even on a large scale and repeatedly over time, appears helpful in determining the duration of the humoral protection elicited by vaccination. These aspects and their implications for the GPP are discussed.
Keywords: Green Pass Policy; SARS-CoV-2; anti-RBD antibody titer; humoral immune response; neutralizing antibodies; vaccine.
Copyright © 2022 Polvere, Parrella, Zerillo, Voccola, Cardinale, D’Andrea, Madera, Stilo, Vito and Zotti.
Conflict of interest statement
Authors SV, SD’A, and RV are employed by the spin-off Genus Biotech srl. Authors TZ and LZ were employed by the spin-off Genus Biotech srl. Authors AP and GC are employed by the company Tecno Bios srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Humoral and Cellular Immune Responses to Vector, Mix-and-Match, or mRNA Vaccines against SARS-CoV-2 and the Relationship between the Two Immune Responses.Microbiol Spectr. 2022 Aug 31;10(4):e0249521. doi: 10.1128/spectrum.02495-21. Epub 2022 Aug 10. Microbiol Spectr. 2022. PMID: 35946811 Free PMC article.
-
SARS-CoV-2 S1 Subunit Booster Vaccination Elicits Robust Humoral Immune Responses in Aged Mice.Microbiol Spectr. 2023 Jun 15;11(3):e0436322. doi: 10.1128/spectrum.04363-22. Epub 2023 May 10. Microbiol Spectr. 2023. PMID: 37162333 Free PMC article.
-
Interdependencies of cellular and humoral immune responses in heterologous and homologous SARS-CoV-2 vaccination.Allergy. 2022 Aug;77(8):2381-2392. doi: 10.1111/all.15247. Epub 2022 Feb 16. Allergy. 2022. PMID: 35124800 Free PMC article.
-
Longevity of immunity following COVID-19 vaccination: a comprehensive review of the currently approved vaccines.Hum Vaccin Immunother. 2022 Nov 30;18(5):2037384. doi: 10.1080/21645515.2022.2037384. Epub 2022 Apr 13. Hum Vaccin Immunother. 2022. PMID: 35417285 Free PMC article. Review.
-
Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
Cited by
-
Humoral Immunity across the SARS-CoV-2 Spike after Sputnik V (Gam-COVID-Vac) Vaccination.Antibodies (Basel). 2024 May 11;13(2):41. doi: 10.3390/antib13020041. Antibodies (Basel). 2024. PMID: 38804309 Free PMC article.
-
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies.Int J Mol Sci. 2023 Mar 10;24(6):5352. doi: 10.3390/ijms24065352. Int J Mol Sci. 2023. PMID: 36982424 Free PMC article. Review.
-
T-cell immunity to SARS-CoV-2: what if the known best is not the optimal course for the long run? Adapting to evolving targets.Front Immunol. 2023 Jun 14;14:1133225. doi: 10.3389/fimmu.2023.1133225. eCollection 2023. Front Immunol. 2023. PMID: 37388738 Free PMC article. Review.
-
Humoral immune response after SARS-CoV-2 vaccination in cladribine-treated multiple sclerosis patients.Vaccine X. 2024 Jan 20;16:100445. doi: 10.1016/j.jvacx.2024.100445. eCollection 2024 Jan. Vaccine X. 2024. PMID: 38304878 Free PMC article.
-
An Overview of Current Accomplishments and Gaps of COVID-19 Vaccine Platforms and Considerations for Next Generation Vaccines.J Pharm Sci. 2023 May;112(5):1345-1350. doi: 10.1016/j.xphs.2023.01.019. Epub 2023 Feb 2. J Pharm Sci. 2023. PMID: 36736775 Free PMC article. Review.
References
-
- European Medicines Agency . Authorized COVID-19 Vaccines. Available at: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr... (Accessed November, 23rd 2021).
-
- Food and Drug Administration . COVID-19 Vaccines Authorized for Emergency Use or FDA-Approved. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... (Accessed November, 23rd 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

