Effects of IL-38 on Macrophages and Myocardial Ischemic Injury
- PMID: 35634320
- PMCID: PMC9136064
- DOI: 10.3389/fimmu.2022.894002
Effects of IL-38 on Macrophages and Myocardial Ischemic Injury
Abstract
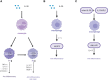
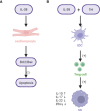
Macrophages play an important role in clearing necrotic myocardial tissues, myocardial ischemia-reperfusion injury, and ventricular remodeling after myocardial infarction. M1 macrophages not only participate in the inflammatory response in myocardial tissues after infarction, which causes heart damage, but also exert a protective effect on the heart during ischemia. In contrast, M2 macrophages exhibit anti-inflammatory and tissue repair properties by inducing the production of high levels of anti-inflammatory cytokines and fibro-progenitor cells. Interleukin (IL)-38, a new member of the IL-1 family, has been reported to modulate the IL-36 signaling pathway by playing a role similar to that of the IL-36 receptor antagonist, which also affects the production and secretion of macrophage-related inflammatory factors that play an anti-inflammatory role. IL-38 can relieve myocardial ischemia-reperfusion injury by promoting the differentiation of M1 macrophages into M2 macrophages, inhibit the activation of NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) inflammasome, and increase the secretion of anti-inflammatory cytokines, such as IL-10 and transforming growth factor-β. The intact recombinant IL-38 can also bind to interleukin 1 receptor accessory protein-like 1 (IL-1RAPL1) to activate the c-jun N-terminal kinase/activator protein 1 (JNK/AP1) pathway and increase the production of IL-6. In addition, IL-38 regulates dendritic cell-induced cardiac regulatory T cells, thereby regulating macrophage polarization and improving ventricular remodeling after myocardial infarction. Accordingly, we speculated that IL-38 and macrophage regulation may be therapeutic targets for ameliorating myocardial ischemic injury and ventricular remodeling after myocardial infarction. However, the specific mechanism of the IL-38 action warrants further investigation.
Keywords: inflammation; interleukin-38; macrophages; myocardial infarction; myocardial ischemia–reperfusion injury; ventricular remodeling.
Copyright © 2022 Li, Ding, Peng, Yu, Pan, Cai, Dong, Zhong, Zhu, Yu and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

