Helix-Loop-Helix Proteins in Adaptive Immune Development
- PMID: 35634342
- PMCID: PMC9134016
- DOI: 10.3389/fimmu.2022.881656
Helix-Loop-Helix Proteins in Adaptive Immune Development
Abstract
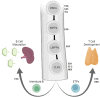
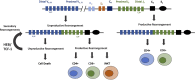
The E/ID protein axis is instrumental for defining the developmental progression and functions of hematopoietic cells. The E proteins are dimeric transcription factors that activate gene expression programs and coordinate changes in chromatin organization. Id proteins are antagonists of E protein activity. Relative levels of E/Id proteins are modulated throughout hematopoietic development to enable the progression of hematopoietic stem cells into multiple adaptive and innate immune lineages including natural killer cells, B cells and T cells. In early progenitors, the E proteins promote commitment to the T and B cell lineages by orchestrating lineage specific programs of gene expression and regulating VDJ recombination of antigen receptor loci. In mature B cells, the E/Id protein axis functions to promote class switch recombination and somatic hypermutation. E protein activity further regulates differentiation into distinct CD4+ and CD8+ T cells subsets and instructs mature T cell immune responses. In this review, we discuss how the E/Id proteins define the adaptive immune system lineages, focusing on their role in directing developmental gene programs.
Keywords: B cell development; E proteins; HLH; Id proteins; T cell development; VDJ recombination; hematopoiesis; lymphopoiesis.
Copyright © 2022 Aubrey, Warburg and Murre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

