The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia
- PMID: 35634504
- PMCID: PMC9134856
- DOI: 10.3389/fendo.2022.873662
The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia
Abstract
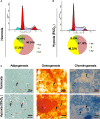
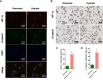
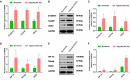
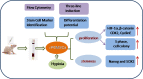
This study aimed to address the dilemma of low peripheral blood-derived mesenchymal stromal cell (PBMSC) activity and reduced phenotype in bone or cartilage tissue engineering. Rat PBMSCs (rPBMSCs) were obtained by density gradient centrifugation, and stromal cell characteristics were confirmed by flow cytometry (FCM) and multi-differentiation potential induction experiments. Cell growth curve, viability experiments, and clone formation experiments were performed by [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (MTS) and cell counting, and the cell cycle was confirmed by cell FCM. The proliferation signal pathway and stemness-related proteins were detected by molecular methods including Western blot and real-time polymerase chain reaction. CD73, CD90, and CD105 were highly expressed, and CD14, CD19, CD34, CD45, and HLA-DR were barely expressed in rPBMSCs. rPBMSCs possessed the potential to differentiate into chondrocytes, adipocytes, and osteoblasts under their respective induction conditions. Cell growth curve and viability experiments were performed under hypoxic conditions: 19% O2, 5% O2, and 1% O2. Specifically, 5% O2 accelerated the proliferation and expression of the stemness of PBMSCs. Cycle experiments proved that hypoxia promoted the cell transition from the G1 phase to the S phase. Molecular experiments confirmed that 5% O2 hypoxia significantly elevated the expressions of hypoxia-inducible factor 1α and β-catenin and simultaneously the expressions of cycle-related genes including CyclinE/CDK2 and stemness-related genes including Nanog and SOX2. The appropriate concentration of hypoxia (i.e., 5% O2) enhanced the proliferation and stemness of rPBMSCs and increased the multidirectional differentiation potential of stromal cells. The proposed culture method could improve the viability and maintain the phenotype of rPBMSCs in cartilage or bone tissue engineering.
Keywords: HIF-1α; hypoxia; peripheral blood-derived mesenchymal stromal cells (PBMSCs); proliferation; stemness.
Copyright © 2022 Wang, Zhu, Yu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hypoxia with Wharton's jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells.Stem Cell Res Ther. 2018 Jun 13;9(1):158. doi: 10.1186/s13287-018-0902-5. Stem Cell Res Ther. 2018. PMID: 29895317 Free PMC article.
-
[Enrichment and Biological Characteristics of Peripheral Blood-derived Mesenchymal Stem Cells in Rats].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015 Apr;23(2):506-11. doi: 10.7534/j.issn.1009-2137.2015.02.041. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015. PMID: 25948214 Chinese.
-
Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression.Connect Tissue Res. 2019 Nov;60(6):583-596. doi: 10.1080/03008207.2019.1611792. Epub 2019 May 28. Connect Tissue Res. 2019. PMID: 31035811
-
Hypoxia enhances buffalo adipose-derived mesenchymal stem cells proliferation, stemness, and reprogramming into induced pluripotent stem cells.J Cell Physiol. 2019 Aug;234(10):17254-17268. doi: 10.1002/jcp.28342. Epub 2019 Feb 25. J Cell Physiol. 2019. PMID: 30805934
-
Isolation and Characterization of Rat Mesenchymal Stem Cells Derived from Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood.Cells Tissues Organs. 2015 -2016;201(6):412-422. doi: 10.1159/000445855. Epub 2016 Jun 1. Cells Tissues Organs. 2015. PMID: 27246344
Cited by
-
Regulation Mechanisms and Maintenance Strategies of Stemness in Mesenchymal Stem Cells.Stem Cell Rev Rep. 2024 Feb;20(2):455-483. doi: 10.1007/s12015-023-10658-3. Epub 2023 Nov 27. Stem Cell Rev Rep. 2024. PMID: 38010581 Review.
-
HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases.Cells. 2022 Nov 10;11(22):3552. doi: 10.3390/cells11223552. Cells. 2022. PMID: 36428981 Free PMC article. Review.
-
Hypoxia Promotes the In Vitro Proliferation of Buffalo Spermatogonial Cells by Increasing Lactate and H3K18la Lactylation Levels.Cells. 2025 Jun 3;14(11):832. doi: 10.3390/cells14110832. Cells. 2025. PMID: 40498008 Free PMC article.
-
The Influence of Intervertebral Disc Microenvironment on the Biological Behavior of Engrafted Mesenchymal Stem Cells.Stem Cells Int. 2022 Nov 7;2022:8671482. doi: 10.1155/2022/8671482. eCollection 2022. Stem Cells Int. 2022. PMID: 36387746 Free PMC article. Review.
-
The issue of heterogeneity of MSC-based advanced therapy medicinal products-a review.Front Cell Dev Biol. 2024 Jul 26;12:1400347. doi: 10.3389/fcell.2024.1400347. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39129786 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

