Small Noncoding RNAome Changes During Human Bone Marrow Mesenchymal Stem Cells Senescence In Vitro
- PMID: 35634512
- PMCID: PMC9135970
- DOI: 10.3389/fendo.2022.808223
Small Noncoding RNAome Changes During Human Bone Marrow Mesenchymal Stem Cells Senescence In Vitro
Abstract
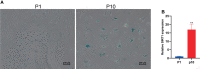
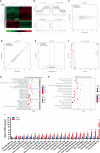
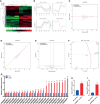
Bone marrow mesenchymal stem cells (BMSCs) have been used in stem cell-based therapy for various diseases due to their self-renewing ability and differentiation potential to various types of cells and immunoprivileged properties. However, the proliferation capability and functionality of BMSCs are known to decline with aging, which severely limits the extensive applications of BMSC-based therapies. To date, the exact mechanism involved in the cellular senescence of BMSCs remains unclear. RNA is thought to be the initial molecular form of life on earth. It also acts as a transmitter and important regulator of genetic information expression. There are many kinds of small noncoding RNAs with different functions in cells that regulate important life activity processes in multiple dimensions, including development process, gene expression, genomic stability, and cellular senescence. In this study, a replicative senescence model of hBMSCs was established and the expression changes of small noncoding RNAs during senescence were detected by small RNA high-throughput sequencing analysis and qPCR. Small RNA sequencing results showed that there were significant differences in the expression of 203 miRNAs, 46 piRNAs, 63 snoRNAs, 12 snRNAs, and 7 rasiRNAs. The results of qPCR, which was performed for the verification of the sequencing results, showed that there were significant differences in the expression of 24 miRNAs, 34 piRNAs, 34 snoRNAs, and 2 snRNAs. These findings might provide a novel insight into hBMSC senescence and contribute to the development of new targeting senescence strategies.
Keywords: bone marrow mesenchymal stem cells; miRNA; piRNA; senescence; snRNA; snoRNA.
Copyright © 2022 Xiao, Peng, Li, Zhou, Ma, Dai, Yuan, Chen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and Senescence of Human Marrow Stromal Cells in Culture: A Simple Colony-Forming Assay Identifies Samples With the Greatest Potential to Propagate and Differentiate. Br J Haematol (1999) 107:275–81. doi: 10.1046/j.1365-2141.1999.01715.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

