Enzymatic glycan remodeling-metal free click (GlycoConnect™) provides homogenous antibody-drug conjugates with improved stability and therapeutic index without sequence engineering
- PMID: 35634725
- PMCID: PMC9154768
- DOI: 10.1080/19420862.2022.2078466
Enzymatic glycan remodeling-metal free click (GlycoConnect™) provides homogenous antibody-drug conjugates with improved stability and therapeutic index without sequence engineering
Abstract
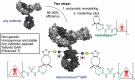
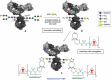
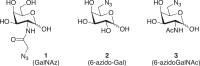
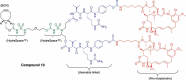
Antibody-drug conjugates (ADCs) are increasingly powerful medicines for targeted cancer therapy. Inspired by the trend to further improve their therapeutic index by generation of homogenous ADCs, we report here how the clinical-stage GlycoConnect™ technology uses the globally conserved N-glycosylation site to generate stable and site-specific ADCs based on enzymatic remodeling and metal-free click chemistry. We demonstrate how an engineered endoglycosidase and a native glycosyl transferase enable highly efficient, one-pot glycan remodeling, incorporating a novel sugar substrate 6-azidoGalNAc. Metal-free click attachment of an array of cytotoxic payloads was highly optimized, in particular by inclusion of anionic surfactants. The therapeutic potential of GlycoConnect™, in combination with HydraSpace™ polar spacer technology, was compared to that of Kadcyla® (ado-trastuzumab emtansine), showing significantly improved efficacy and tolerability.
Keywords: Antibody-drug conjugates (ADCs); chemoenzymatic; glycan remodeling; metal-free click chemistry; non-genetic; therapeutic index.
Conflict of interest statement
All authors are employed by Synaffix BV or were employed at the time of contribution to the scientific work described.
Figures






References
-
- Lambert JM, van Delft FL.. Introduction to antibody-drug conjugates. In: van Delft FL, Lambert JM, editors. Chemical linkers inantibody-drugconjugates, Royal Society of Chemistry; 2021, chapter 1. p. 1–10. doi:10.1039/9781839165153. - DOI
-
- Thurston DE, Jackson PJM, editors. Cytotoxic payloads for antibody–drug conjugates.. Royal Society of Chemistry; 2019. doi:10.1039/9781788012898. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
