The roles of highly conserved, non-catalytic residues in class A β-lactamases
- PMID: 35634774
- PMCID: PMC9112487
- DOI: 10.1002/pro.4328
The roles of highly conserved, non-catalytic residues in class A β-lactamases
Abstract
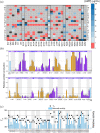
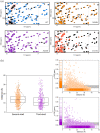
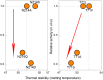
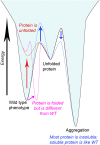
Evolution minimizes the number of highly conserved amino acid residues in proteins to ensure evolutionary robustness and adaptability. The roles of all highly conserved, non-catalytic residues, 11% of all residues, in class A β-lactamase were analyzed by studying the effect of 146 mutations on in cell and in vitro activity, folding, structure, and stability. Residues around the catalytic residues (second shell) contribute to fine-tuning of the active site structure. Mutations affect the structure over the entire active site and can result in stable but inactive protein. Conserved residues farther away (third shell) ensure a favorable balance of folding versus aggregation or stabilize the folded form over the unfolded state. Once folded, the mutant enzymes are stable and active and show only localized structural effects. These residues are found in clusters, stapling secondary structure elements. The results give an integral picture of the different roles of essential residues in enzymes.
Keywords: BlaC; conserved residues; protein evolution; β-Lactamase.
© 2022 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
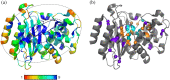


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

