Inhaled silica nanoparticles cause chronic kidney disease in rats
- PMID: 35635324
- PMCID: PMC9236867
- DOI: 10.1152/ajprenal.00021.2022
Inhaled silica nanoparticles cause chronic kidney disease in rats
Abstract
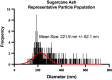
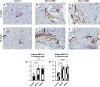
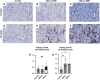
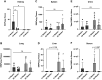
Silica nanoparticles (SiNPs) released during the burning of sugarcane have been postulated to have a role in chronic kidney disease of unknown etiology. We tested the hypothesis that pristine SiNPs of the size present in sugarcane might cause chronic kidney injury when administered through the lung in rats. We administered 200- or 300-nm amorphous SiNPs twice weekly (4 mg/dose), or vehicle by oropharyngeal aspiration for 13 wk to rats followed by euthanasia after an additional 13 wk (26 wk total). Tissues were evaluated for the presence of SiNPs and evidence of histological injury. Both sizes of SiNPs caused kidney damage, with early tubular injury and inflammation (at week 13) that continued to inflammation and chronic fibrosis at week 26 despite discontinuation of the SiNP administration. Both sizes of SiNPs caused local inflammation in the lung and kidney and were detected in the serum and urine at week 13, and the 200-nm particles were also localized to the kidney with no evidence of retention of the 300-nm particles. At week 26, there was some clearance of the 200-nm silica from the kidneys, and urinary levels of SiNPs were reduced but still significant in both 200- and 300 nm-exposed rats. In conclusion, inhaled SiNPs cause chronic kidney injury that progresses despite stopping the SiNP administration. These findings support the hypothesis that human exposure to amorphous silica nanoparticles found in burned sugarcane fields could have a participatory role in chronic kidney disease of unknown etiology.NEW & NOTEWORTHY Inhalation of silica nanoparticles (SiNPs) released during the burning of sugarcane has been postulated to have a role in chronic kidney disease of unknown etiology (CKDu). We administered 200- and 300-nm amorphous SiNPs to rats by aspiration and observed kidney damage with tubular injury and inflammation that persisted even after stopping the SiNP exposure. These findings support the hypothesis that human exposure to SiNPs found in sugarcane ash could have a participatory role CKDu.
Keywords: Mesoamerican nephropathy; chronic kidney disease of unknown etiology; silica nanoparticles; sugarcane.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

