Antiemetic prophylaxis for chemoradiotherapy-induced nausea and vomiting in locally advanced head and neck squamous cell carcinoma: a prospective phase II trial
- PMID: 35635557
- PMCID: PMC9149669
- DOI: 10.1007/s00066-022-01958-7
Antiemetic prophylaxis for chemoradiotherapy-induced nausea and vomiting in locally advanced head and neck squamous cell carcinoma: a prospective phase II trial
Abstract
Background: There is sparse research reporting effective interventions for preventing nausea and emesis caused by concurrent chemoradiotherapy (CCRT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC).
Methods: Treatment-naïve LA-HNSCC patients received intensity-modulated radiotherapy with concomitant cisplatin 100 mg/m2 (33 mg/m2/days [d]1-3) every 3 weeks for two cycles. All patients were given oral aprepitant 125 mg once on d1, then 80 mg once on d2-5; ondansetron 8 mg once on d1; and dexamethasone 12 mg once on d1, then 8 mg on d2-5. The primary endpoint was complete response (CR). Pursuant to δ = 0.2 and α = 0.05, the expected CR rate was 80%.
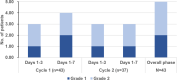
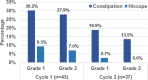
Results: A total of 43 patients with LA-HNSCC were enrolled. The median age was 53 years, and 86.0% were male. All patients received radiotherapy and 86.0% of patients completed both cycles as planned. The overall CR rate was 86.0% (95% confidence interval [CI]: 72.1-94.7). The CR rates for cycles 1 and 2 were 88.4% (95% CI: 74.9-96.1) and 89.2% (95% CI: 74.6-97.0). The complete protection rate in the overall phase was 72.1% (95% CI: 56.3-84.7). The emesis-free and nausea-free responses in the overall phase were 88.4% (95% CI: 74.9-96.1) and 60.5% (95% CI: 44.4-75.0), respectively. The adverse events related to antiemetics were constipation (65.1%) and hiccups (16.3%), but both were grade 1-2. There was no grade 4 or 5 treatment-related toxicity with antiemetic usage.
Conclusion: The addition of aprepitant into ondansetron and dexamethasone provided effective protection from nausea and emesis in patients with LA-HNSCC receiving radiotherapy and concomitant high-dose cisplatin chemotherapy.
Keywords: Antiemetic regimen; Concomitant chemotherapy; Intensity-modulated radiotherapy; Side effects; Complete response.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany.
Conflict of interest statement
Z. Wang, W. Liu, J. Zhang, X. Chen, J. Wang, K. Wang, Y. Qu, X. Huang, J. Luo, J. Xiao, G. Xu, L. Gao, J. Yi, and Y. Zhang declare that they have no competing interests.
Figures


Similar articles
-
Addition of the Neurokinin-1-Receptor Antagonist (RA) Aprepitant to a 5-Hydroxytryptamine-RA and Dexamethasone in the Prophylaxis of Nausea and Vomiting Due to Radiation Therapy With Concomitant Cisplatin.Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):1101-1107. doi: 10.1016/j.ijrobp.2015.04.037. Epub 2015 Apr 28. Int J Radiat Oncol Biol Phys. 2015. PMID: 26059352 Clinical Trial.
-
The Efficacy of Palonosetron Plus Dexamethasone in Preventing Chemoradiotherapy-induced Nausea and Emesis in Patients Receiving Daily Low-dose Cisplatin-based Concurrent Chemoradiotherapy for Uterine Cervical Cancer: A Phase II Study.Am J Clin Oncol. 2017 Apr;40(2):118-121. doi: 10.1097/COC.0000000000000117. Am J Clin Oncol. 2017. PMID: 25144265 Clinical Trial.
-
The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomised, placebo-controlled phase III clinical trials.Eur J Cancer. 2004 Feb;40(3):403-10. Eur J Cancer. 2004. PMID: 14746859 Clinical Trial.
-
Ondansetron. An update of its therapeutic use in chemotherapy-induced and postoperative nausea and vomiting.Drugs. 1993 Jun;45(6):931-952. doi: 10.2165/00003495-199345060-00006. Drugs. 1993. PMID: 7691500 Review.
-
Aprepitant: a review of its use in the prevention of chemotherapy-induced nausea and vomiting.Drugs. 2004;64(7):777-94. doi: 10.2165/00003495-200464070-00013. Drugs. 2004. PMID: 15025555 Review.
Cited by
-
Nonsurgical Treatment Strategies for Elderly Head and Neck Cancer Patients: An Emerging Subject Worldwide.Cancers (Basel). 2022 Nov 19;14(22):5689. doi: 10.3390/cancers14225689. Cancers (Basel). 2022. PMID: 36428780 Free PMC article. Review.
-
The bibliometric analysis of research on traditional Chinese medicine regulating gut microbiota for cancer treatment from 2014 to 2024.Hereditas. 2025 Jun 3;162(1):94. doi: 10.1186/s41065-025-00456-x. Hereditas. 2025. PMID: 40462156 Free PMC article.
-
Dexamethasone to prednisolone rotation relieved hiccups in colorectal cancer patient continuing teleworking during anticancer therapy.Clin Case Rep. 2023 Jun 20;11(6):e7367. doi: 10.1002/ccr3.7367. eCollection 2023 Jun. Clin Case Rep. 2023. PMID: 37351354 Free PMC article.
-
2023 Updated MASCC/ESMO Consensus Recommendations: prevention of radiotherapy- and chemoradiotherapy-induced nausea and vomiting.Support Care Cancer. 2023 Dec 15;32(1):26. doi: 10.1007/s00520-023-08226-z. Support Care Cancer. 2023. PMID: 38097904 Free PMC article. Review.
-
Harnessing the power of traditional Chinese medicine monomers and compound prescriptions to boost cancer immunotherapy.Front Immunol. 2023 Nov 15;14:1277243. doi: 10.3389/fimmu.2023.1277243. eCollection 2023. Front Immunol. 2023. PMID: 38035069 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

