Inhibition of nuclear deacetylase Sirtuin-1 induces mitochondrial acetylation and calcium overload leading to cell death
- PMID: 35636016
- PMCID: PMC9142701
- DOI: 10.1016/j.redox.2022.102334
Inhibition of nuclear deacetylase Sirtuin-1 induces mitochondrial acetylation and calcium overload leading to cell death
Abstract
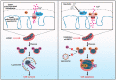
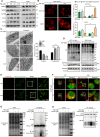
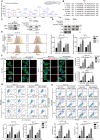
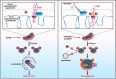
Sirtuin-1 (SIRT1) is a critical nuclear deacetylase that participates in a wide range of biological processes. We hereby employed quantitative acetyl-proteomics to globally reveal the landscape of SIRT1-dependent acetylation in colorectal cancer (CRC) cells stimulated by specific SIRT1 inhibitor Inauhzin (INZ). We strikingly observed that SIRT1 inhibition enhances protein acetylation levels, with the multisite-acetylated proteins (acetyl sites >4/protein) mainly enriched in mitochondria. INZ treatment increases mitochondrial fission and depolarization in CRC cells. The acetylation of mitochondrial proteins promoted by SIRT1 inhibition prevents the recruitment of ubiquitin and LC3 for mitophagic degradation. We then found that, SIRT1 inhibition increases the acetylation of mitochondrial calcium uniporter (MCU) at residue K332, resulting in mitochondrial Ca2+ overload and depolarization, and ultimately CRC apoptosis. Arginine substitution of the K332 (K332R) dramatically decreases the mitochondrial Ca2+ influx, mitochondrial membrane potential loss and ROS burst induced by INZ. This finding uncovers a non-canonical role of SIRT1 in regulating mitochondrial function and implicates a possible way for anticancer intervention through SIRT1 inhibition.
Keywords: Acetylation; Calcium; Inauhzin; MCU; Mitochondria; SIRT1.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Wang C., Wang Y., Shen L. Mitochondrial proteins in heart failure: the role of deacetylation by SIRT3. Pharmacol. Res. 2021;172 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

