Common risk variants for epilepsy are enriched in families previously targeted for rare monogenic variant discovery
- PMID: 35636315
- PMCID: PMC9156876
- DOI: 10.1016/j.ebiom.2022.104079
Common risk variants for epilepsy are enriched in families previously targeted for rare monogenic variant discovery
Abstract
Background: The epilepsies are highly heritable conditions that commonly follow complex inheritance. While monogenic causes have been identified in rare familial epilepsies, most familial epilepsies remain unsolved. We aimed to determine (1) whether common genetic variation contributes to familial epilepsy risk, and (2) whether that genetic risk is enriched in familial compared with non-familial (sporadic) epilepsies.
Methods: Using common variants derived from the largest epilepsy genome-wide association study, we calculated polygenic risk scores (PRS) for patients with familial epilepsy (n = 1,818 from 1,181 families), their unaffected relatives (n = 771), sporadic patients (n = 1,182), and population controls (n = 15,929). We also calculated separate PRS for genetic generalised epilepsy (GGE) and focal epilepsy. Statistical analyses used mixed-effects regression models to account for familial relatedness, sex, and ancestry.
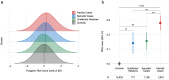
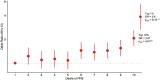
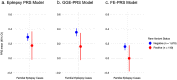
Findings: Patients with familial epilepsies had higher epilepsy PRS compared to population controls (OR 1·20, padj = 5×10-9), sporadic patients (OR 1·11, padj = 0.008), and their own unaffected relatives (OR 1·12, padj = 0.01). The top 1% of the PRS distribution was enriched 3.8-fold for individuals with familial epilepsy when compared to the lowest decile (padj = 5×10-11). Familial PRS enrichment was consistent across epilepsy type; overall, polygenic risk was greatest for the GGE clinical group. There was no significant PRS difference in familial cases with established rare variant genetic etiologies compared to unsolved familial cases.
Interpretation: The aggregate effects of common genetic variants, measured as polygenic risk scores, play an important role in explaining why some families develop epilepsy, why specific family members are affected while their relatives are not, and why families manifest specific epilepsy types. Polygenic risk contributes to the complex inheritance of the epilepsies, including in individuals with a known genetic etiology.
Funding: National Health and Medical Research Council of Australia, National Institutes of Health, American Academy of Neurology, Thomas B and Jeannette E Laws McCabe Fund, Mirowski Family Foundation.
Keywords: Common genetic variation; Epilepsy genetics; Familial epilepsies; Polygenic risk scores.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Samuel Berkovic has received grants from UCB Pharma, Eisai, SciGen; consulting fees from Praxis Precision Medicines, Sequiris; honoraria from Eisai; has a patent for SCN1A testing held by Bionomics Inc licensed to Athena Diagnostics and Genetics Technologies Ltd. Ingrid Scheffer has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, Chiesi, Encoded Therapeutics, Knopp Biosciences and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin and Eisai; has served as an investigator for Zogenix, Zynerba, Ultragenyx, GW Pharma, UCB, Eisai, Xenon Pharmaceuticals, Anavex Life Sciences, Ovid Therapeutics, Epigenyx, Encoded Therapeutics and Marinus; and has consulted for Zynerba Pharmaceuticals, Atheneum Partners, Ovid Therapeutics, Care Beyond Diagnosis, Epilepsy Consortium and UCB; and is a Non-Executive Director of Bellberry Ltd. She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies; has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID:2012-009). David Whiteman has received speaker fees from Pierre Fabre. Melanie Bahlo has received payment for thesis examination from University of Melbourne, University of Sydney, University of Western Australia; has received support for attending conferences: Genemappers, International Conference on Familial Cortical Myoclonic Tremor Epilepsy and Repeat Expansion Diseases, Lorne Genome, Bioinforsummer, Australian Academy of Science Australia-China symposium on precision medicine; has served as an advisor for ALADIN, Kinghorn Sequencing Center, Murdoch Children's Research Institute, Viertel Foundation; is a committee member for Australian Academy of Health and Medical Sciences, American Epilepsy Society Basic Sciences, Gen V Bioresource Genetics. Costin Leu received conference travel support from the International Epilepsy Congress/ILAE. Ingo Helbig received travel support from the Americal Epilepsy Society. Heather Mefford has received support from CURE Epilepsy and sits on the American Society of Human Genetics Board of Directors. Lynette Sadleir has received consulting fees from the Epilepsy Consortium, Zynerba; is an advisor to Eisai; and is treasurer for the New Zealand League Against Epilepsy.
Figures

References
-
- Annegers J.F., Hauser W.A., Anderson V.E., Kurland LT. The risks of seizure disorders among relatives of patients with childhood onset epilepsy. Neurology. 1982;32:174–179. - PubMed
-
- Lennox W.G. The heredity of epilepsy as told by relatives and twins. J Am Med Assoc. 1951;146:529–536. - PubMed
-
- Steinlein O.K., Mulley J.C., Propping P., et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–203. - PubMed
-
- Perucca P., Bahlo M., Berkovic S.F. The genetics of epilepsy. Annu Rev Genom Hum Genet. 2020;21:205–230. - PubMed

