Global guidelines for breast cancer screening: A systematic review
- PMID: 35636342
- PMCID: PMC9142711
- DOI: 10.1016/j.breast.2022.04.003
Global guidelines for breast cancer screening: A systematic review
Abstract
Objectives: Breast cancer screening guidelines could provide valuable tools for clinical decision making by reviewing the available evidence and providing recommendations. Little information is known about how many countries have issued breast cancer screening guidelines and the differences among existing guidelines. We systematically reviewed current guidelines and summarized corresponding recommendations, to provide references for good clinical practice in different countries.
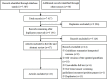
Methods: Systematic searches of MEDLINE, EMBASE, Web of Science, and Scopus from inception to March 27th, 2021 were conducted and supplemented by reviewing the guideline development organizations. The quality of screening guidelines was assessed from six domains of the Appraisal of Guidelines for Research and Evaluation Ⅱ (AGREE Ⅱ) instrument by two appraisers. The basic information and recommendations of the issued guidelines were extracted and summarized.
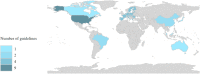
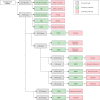
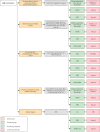
Results: A total of 23 guidelines issued between 2010 and 2021 in 11 countries or regions were identified for further review. The content and quality varied across the guidelines. The average AGREE Ⅱ scores of the guidelines ranged from 33.3% to 87.5%. The highest domain score was "clarity of presentation" while the domain with the lowest score was "applicability". For average-risk women, most of the guidelines recommended mammographic screening for those aged 40-74 years, specifically, those aged 50-69 years were regarded as the optimal age group for screening. Nine of 23 guidelines recommended against an upper age limit for breast cancer screening. Mammography (MAM) was recommended as the primary screening modality for average-risk women by all included guidelines. Most guidelines suggested annual or biennial mammographic screening. Risk factors of breast cancer identified in the guidelines mainly fell within five categories which could be broadly summarized as the personal history of pre-cancerous lesions and/or breast cancer; the family history of breast cancer; the known genetic predisposition of breast cancer; the history of mantle or chest radiation therapy; and dense breasts. For women at higher risk, there was a consensus among most guidelines that annual MAM or annual magnetic resonance imaging (MRI) should be given, and the screening should begin earlier than the average-risk group.
Conclusions: The majority of 23 included international guidelines were issued by developed countries which contained roughly the same but not identical recommendations on breast cancer screening age, methods, and intervals. Most guidelines recommended annual or biennial mammographic screening between 40 and 74 years for average-risk populations and annual MAM or annual MRI starting from a younger age for high-risk populations. Current guidelines varied in quality and increased efforts are needed to improve the methodological quality of guidance documents. Due to lacking clinical practice guidelines tailored to different economic levels, low- and middle-income countries (LMICs) should apply and implement the evidence-based guidelines with higher AGREE Ⅱ scores considering local adaption.
Keywords: Breast neoplasms; Guideline; Screening; Systematic review.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures

References
-
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Available at: https://gco.iarc.fr/today/. [accessed 2021-05-01].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

