Brain motor and fear circuits regulate leukocytes during acute stress
- PMID: 35636458
- PMCID: PMC9798885
- DOI: 10.1038/s41586-022-04890-z
Brain motor and fear circuits regulate leukocytes during acute stress
Abstract
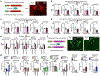
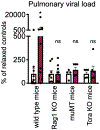
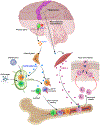
The nervous and immune systems are intricately linked1. Although psychological stress is known to modulate immune function, mechanistic pathways linking stress networks in the brain to peripheral leukocytes remain poorly understood2. Here we show that distinct brain regions shape leukocyte distribution and function throughout the body during acute stress in mice. Using optogenetics and chemogenetics, we demonstrate that motor circuits induce rapid neutrophil mobilization from the bone marrow to peripheral tissues through skeletal-muscle-derived neutrophil-attracting chemokines. Conversely, the paraventricular hypothalamus controls monocyte and lymphocyte egress from secondary lymphoid organs and blood to the bone marrow through direct, cell-intrinsic glucocorticoid signalling. These stress-induced, counter-directional, population-wide leukocyte shifts are associated with altered disease susceptibility. On the one hand, acute stress changes innate immunity by reprogramming neutrophils and directing their recruitment to sites of injury. On the other hand, corticotropin-releasing hormone neuron-mediated leukocyte shifts protect against the acquisition of autoimmunity, but impair immunity to SARS-CoV-2 and influenza infection. Collectively, these data show that distinct brain regions differentially and rapidly tailor the leukocyte landscape during psychological stress, therefore calibrating the ability of the immune system to respond to physical threats.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures







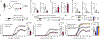
References
-
- Schiller M, Ben-Shaanan TL & Rolls A Neuronal regulation of immunity: why, how and where. Nat. Rev. Immunol 21, 20–36 (2021). - PubMed
-
- Chervenick PA, Boggs DR, Marsh JC, Cartwright GE & Wintrobe MM Quantitative studies of blood and bone marrow neutrophils in normal mice. Am. J. Physiol 215, 353–360 (1968). - PubMed
-
- Colvin GA et al. Murine marrow cellularity and the concept of stem cell competition: geographic and quantitative determinants in stem cell biology. Leukemia 18, 575–583 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

