Proteasome dysfunction disrupts adipogenesis and induces inflammation via ATF3
- PMID: 35636710
- PMCID: PMC9194453
- DOI: 10.1016/j.molmet.2022.101518
Proteasome dysfunction disrupts adipogenesis and induces inflammation via ATF3
Abstract
Objective: Regulation of proteasomal activity is an essential component of cellular proteostasis and function. This is evident in patients with mutations in proteasome subunits and associated regulators, who suffer from proteasome-associated autoinflammatory syndromes (PRAAS). These patients display lipodystrophy and fevers, which may be partly related to adipocyte malfunction and abnormal thermogenesis in adipose tissue. However, the cell-intrinsic pathways that could underlie these symptoms are unclear. Here, we investigate the impact of two proteasome subunits implicated in PRAAS, Psmb4 and Psmb8, on differentiation, function and proteostasis of brown adipocytes.
Methods: In immortalized mouse brown pre-adipocytes, levels of Psmb4, Psmb8, and downstream effectors genes were downregulated through reverse transfection with siRNA. Adipocytes were differentiated and analyzed with various assays of adipogenesis, lipogenesis, lipolysis, inflammation, and respiration.
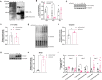
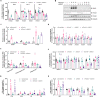
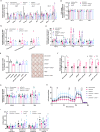
Results: Loss of Psmb4, but not Psmb8, disrupted proteostasis and adipogenesis. Proteasome function was reduced upon Psmb4 loss, but partly recovered by the activation of Nuclear factor, erythroid-2, like-1 (Nfe2l1). In addition, cells displayed higher levels of surrogate inflammation and stress markers, including Activating transcription factor-3 (Atf3). Simultaneous silencing of Psmb4 and Atf3 lowered inflammation and restored adipogenesis.
Conclusions: Our study shows that Psmb4 is required for adipocyte development and function in cultured adipocytes. These results imply that in humans with PSMB4 mutations, PRAAS-associated lipodystrophy is partly caused by disturbed adipogenesis. While we uncover a role for Nfe2l1 in the maintenance of proteostasis under these conditions, Atf3 is a key effector of inflammation and blocking adipogenesis. In conclusion, our work highlights how proteasome dysfunction is sensed and mitigated by the integrated stress response in adipocytes with potential relevance for PRAAS patients and beyond.
Keywords: ATF3; Adipocytes; NFE2L1; PSMB4; Proteasome; Proteostasis; Ubiquitin; brown adipose tissue.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Torrelo A., Patel S., Colmenero I., Gurbindo D., Lendínez F., Hernández A., et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. Journal of the American Academy of Dermatology. 2010 Mar;62(3):489–495. - PubMed
-
- Agarwal A.K., Xing C., DeMartino G.N., Mizrachi D., Hernandez M.D., Sousa A.B., et al. PSMB8 encoding the β5i proteasome subunit is mutated in Joint Contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. The American Journal of Human Genetics. 2010 Dec;87(6):866–872. - PMC - PubMed
-
- Kanazawa N. Nakajo-nishimura syndrome: an autoinflammatory disorder showing pernio-like rashes and progressive partial lipodystrophy. Allergology International. 2012;61(2):197–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

