Laboratory Diagnosis for SARS-CoV-2 Infection
- PMID: 35636903
- PMCID: PMC9141924
- DOI: 10.1016/j.idc.2022.02.002
Laboratory Diagnosis for SARS-CoV-2 Infection
Abstract
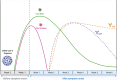
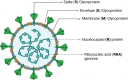
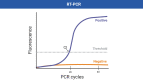
The optimal diagnostic test for SARS-CoV-2 infection should be selected based on a patient's clinical syndrome and presentation in relation to symptom onset. Molecular testing, most often reverse-transcriptase polymerase chain reaction, offers the highest sensitivity and specificity during acute infection, whereas antigen testing can also be useful for acute diagnosis when rapid turnaround of results is necessary or if molecular testing is unavailable. Serologic testing is often reserved for identifying individuals with prior or late COVID-19 infection.
Keywords: Antigen testing; COVID-19; Nucleic acid amplification testing; SARS-CoV-2 diagnostics; Serology.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure S.E. Turbett receives grant support from the Centers for Disease Control and Prevention for COVID-19-related work. She also receives royalties from UpToDate. The remaining authors have nothing to disclose.
Figures
Similar articles
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Sensitivity and specificity of 14 SARS-CoV-2 serological assays and their diagnostic potential in RT-PCR negative COVID-19 infections.Acta Clin Belg. 2022 Apr;77(2):315-320. doi: 10.1080/17843286.2020.1861885. Epub 2020 Dec 22. Acta Clin Belg. 2022. PMID: 33350362 Free PMC article.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
-
Clinical assessment of the Roche SARS-CoV-2 rapid antigen test.Diagnosis (Berl). 2021 Jan 18;8(3):322-326. doi: 10.1515/dx-2020-0154. Print 2021 Aug 26. Diagnosis (Berl). 2021. PMID: 33554511
-
Recent advances in methods for the diagnosis of Corona Virus Disease 2019.J Clin Lab Anal. 2022 Jan;36(1):e24178. doi: 10.1002/jcla.24178. Epub 2021 Dec 17. J Clin Lab Anal. 2022. PMID: 34921443 Free PMC article. Review.
Cited by
-
[Concordance between GeneXpert and Life Real Detection Kit in the diagnosis of acute respiratory infections].Rev Esp Quimioter. 2023 Aug;36(4):427-428. doi: 10.37201/req/136.2022. Epub 2023 May 3. Rev Esp Quimioter. 2023. PMID: 37130333 Free PMC article. Spanish. No abstract available.
-
Evaluation of a novel lyophilized-pellet-based 2019-nCoV nucleic acid detection kit for the diagnosis of COVID-19.PLoS One. 2023 Oct 25;18(10):e0292902. doi: 10.1371/journal.pone.0292902. eCollection 2023. PLoS One. 2023. PMID: 37878570 Free PMC article.
-
Population-Level SARS-CoV-2 RT-PCR Cycle Threshold Values and Their Relationships with COVID-19 Transmission and Outcome Metrics: A Time Series Analysis Across Pandemic Years.Viruses. 2025 Jan 14;17(1):103. doi: 10.3390/v17010103. Viruses. 2025. PMID: 39861892 Free PMC article.
-
Expert consensus on the diagnosis and treatment of severe and critical coronavirus disease 2019 (COVID-19).J Intensive Med. 2022 Aug 26;2(4):199-222. doi: 10.1016/j.jointm.2022.07.001. eCollection 2022 Oct. J Intensive Med. 2022. PMID: 36785648 Free PMC article. No abstract available.
-
Internationally standardized respiratory viral load testing with limited resources: A derivative-of-care calibration strategy for SARS-CoV-2.Influenza Other Respir Viruses. 2024 Jan 23;18(1):e13207. doi: 10.1111/irv.13207. eCollection 2024 Jan. Influenza Other Respir Viruses. 2024. PMID: 38268611 Free PMC article.
References
-
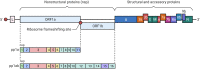
- Greninger AL, Dien Bard J, Colgrove RC, et al. Clinical and infection prevention applications of SARS-CoV-2 genotyping: an IDSA/ASM consensus review document. Clin Infect Dis. Published online November 3, 2021. doi:10.1093/cid/ciab761
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous