NAXE deficiency: A neurometabolic disorder of NAD(P)HX repair amenable for metabolic correction
- PMID: 35637064
- PMCID: PMC9893913
- DOI: 10.1016/j.ymgme.2022.04.003
NAXE deficiency: A neurometabolic disorder of NAD(P)HX repair amenable for metabolic correction
Abstract
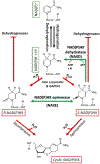
The NAD(P)HX repair system is a metabolite damage repair mechanism responsible for restoration of NADH and NADPH after their inactivation by hydration. Deficiency in either of its two enzymes, NAD(P)HX dehydratase (NAXD) or NAD(P)HX epimerase (NAXE), causes a fatal neurometabolic disorder characterized by decompensations precipitated by inflammatory stress. Clinical findings include rapidly progressive muscle weakness, ataxia, ophthalmoplegia, and motor and cognitive regression, while neuroimaging abnormalities are subtle or nonspecific, making a clinical diagnosis challenging. During stress, nonenzymatic conversion of NAD(P)H to NAD(P)HX increases, and in the absence of repair, NAD(P)H is depleted, and NAD(P)HX accumulates, leading to decompensation; however, the contribution of each to the metabolic derangement is not established. Herein, we summarize the clinical knowledge of NAXE deficiency from 30 cases and lessons learned about disease pathogenesis from cell cultures and model organisms and describe a metabolomics signature obtained by untargeted metabolomics analysis in one case at the time of crisis and after initiation of treatment. Overall, biochemical findings support a model of acute depletion of NAD+, signs of mitochondrial dysfunction, and altered lipidomics. These findings are further substantiated by untargeted metabolomics six months post-crisis showing that niacin supplementation reverses primary metabolomic abnormalities concurrent with improved clinical status.
Keywords: Fever induced encephalopathy; Mitochondrial dysfunction; NAD(+); NAD(P)HX epimerase; NAXE; Neurometabolic disorders; PEBEL1; Pellagra.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics (BG) Laboratories. All authors declare no conflict of interest related to this study.
Figures

References
-
- Ritter M, Buechler C, Boettcher A, Barlage S, Schmitz-Madry A, Orsó E, et al., Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I, Genomics 79 (2002) 693–702, 10.1006/geno.2002.6761. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

