The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases
- PMID: 35637254
- PMCID: PMC9151781
- DOI: 10.1038/s41467-022-30712-x
The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases
Abstract
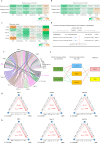
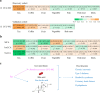
Evidence from human cohorts indicates that chronic insomnia is associated with higher risk of cardiometabolic diseases (CMD), yet whether gut microbiota plays a role is unclear. Here, in a longitudinal cohort (n = 1809), we find that the gut microbiota-bile acid axis may link the positive association between chronic insomnia and CMD. Ruminococcaceae UCG-002 and Ruminococcaceae UCG-003 are the main genera mediating the positive association between chronic insomnia and CMD. These results are also observed in an independent cross-sectional cohort (n = 6122). The inverse associations between those gut microbial biomarkers and CMD are mediated by certain bile acids (isolithocholic acid, muro cholic acid and nor cholic acid). Habitual tea consumption is prospectively associated with the identified gut microbiota and bile acids in an opposite direction compared with chronic insomnia. Our work suggests that microbiota-bile acid axis may be a potential intervention target for reducing the impact of chronic insomnia on cardiometabolic health.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

