Label-free nanofluidic scattering microscopy of size and mass of single diffusing molecules and nanoparticles
- PMID: 35637303
- PMCID: PMC9184284
- DOI: 10.1038/s41592-022-01491-6
Label-free nanofluidic scattering microscopy of size and mass of single diffusing molecules and nanoparticles
Abstract
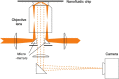
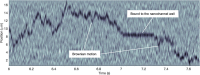
Label-free characterization of single biomolecules aims to complement fluorescence microscopy in situations where labeling compromises data interpretation, is technically challenging or even impossible. However, existing methods require the investigated species to bind to a surface to be visible, thereby leaving a large fraction of analytes undetected. Here, we present nanofluidic scattering microscopy (NSM), which overcomes these limitations by enabling label-free, real-time imaging of single biomolecules diffusing inside a nanofluidic channel. NSM facilitates accurate determination of molecular weight from the measured optical contrast and of the hydrodynamic radius from the measured diffusivity, from which information about the conformational state can be inferred. Furthermore, we demonstrate its applicability to the analysis of a complex biofluid, using conditioned cell culture medium containing extracellular vesicles as an example. We foresee the application of NSM to monitor conformational changes, aggregation and interactions of single biomolecules, and to analyze single-cell secretomes.
© 2022. The Author(s).
Conflict of interest statement
B.S., J.F. and C.L. are founders of Envue Technologies AB, which owns intellectual property related to the described research. J.F. and C.L. are members of the board of Envue Technologies AB. The remaining authors declare no competing interests.
Figures








References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

