Engineering the next generation of cell-based therapeutics
- PMID: 35637318
- PMCID: PMC9149674
- DOI: 10.1038/s41573-022-00476-6
Engineering the next generation of cell-based therapeutics
Abstract
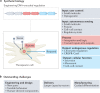
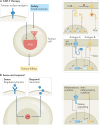
Cell-based therapeutics are an emerging modality with the potential to treat many currently intractable diseases through uniquely powerful modes of action. Despite notable recent clinical and commercial successes, cell-based therapies continue to face numerous challenges that limit their widespread translation and commercialization, including identification of the appropriate cell source, generation of a sufficiently viable, potent and safe product that meets patient- and disease-specific needs, and the development of scalable manufacturing processes. These hurdles are being addressed through the use of cutting-edge basic research driven by next-generation engineering approaches, including genome and epigenome editing, synthetic biology and the use of biomaterials.
© 2022. Springer Nature Limited.
Conflict of interest statement
All authors are inventors on several patents in the field of cell-based therapeutics, biomaterials, genome editing and genetic engineering that are owned by their current or former employers. D.M.S. holds equity in Sigilon Therapeutics and is currently an employee and shareholder of Arbor Bio. H.B. holds equity in Sigilon Therapeutics and is currently an employee and shareholder of Flagship Pioneering. O.V. is co-founder, holds equity in and receives consulting payments from Sigilon Therapeutics, Pana Bio, Avenge Bio and Curada Bio. O.V. has received compensation for consulting from Establishment Labs and Auregen Bio Therapeutics SA. The views presented here should not be considered as endorsements of any specific product or company.
Figures



References
-
- Kamath, A. V. Translational pharmacokinetics and pharmacodynamics of monoclonal antibodies. Drug. Discov. Today Technol. 21–22, 75–83 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

