In utero origin of myelofibrosis presenting in adult monozygotic twins
- PMID: 35637336
- PMCID: PMC9205768
- DOI: 10.1038/s41591-022-01793-4
In utero origin of myelofibrosis presenting in adult monozygotic twins
Abstract
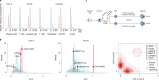
The latency between acquisition of an initiating somatic driver mutation by a single-cell and clinical presentation with cancer is largely unknown. We describe a remarkable case of monozygotic twins presenting with CALR mutation-positive myeloproliferative neoplasms (MPNs) (aged 37 and 38 years), with a clinical phenotype of primary myelofibrosis. The CALR mutation was absent in T cells and dermal fibroblasts, confirming somatic acquisition. Whole-genome sequencing lineage tracing revealed a common clonal origin of the CALR-mutant MPN clone, which occurred in utero followed by twin-to-twin transplacental transmission and subsequent similar disease latency. Index sorting and single-colony genotyping revealed phenotypic hematopoietic stem cells (HSCs) as the likely MPN-propagating cell. Furthermore, neonatal blood spot analysis confirmed in utero origin of the JAK2V617F mutation in a patient presenting with polycythemia vera (aged 34 years). These findings provide a unique window into the prolonged evolutionary dynamics of MPNs and fitness advantage exerted by MPN-associated driver mutations in HSCs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- 28051/CRUK_/Cancer Research UK/United Kingdom
- 26988/CRUK_/Cancer Research UK/United Kingdom
- C42639/A26988/CRUK_/Cancer Research UK/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/L006340/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12025/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- C130623/A249471/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00029/7/MRC_/Medical Research Council/United Kingdom
- MR/M00919X/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/MRC_/Medical Research Council/United Kingdom
- G0902418/MRC_/Medical Research Council/United Kingdom
- 29034/CRUK_/Cancer Research UK/United Kingdom
- 30721/CRUK_/Cancer Research UK/United Kingdom
- 203141/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/R006237/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/5/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

