Polarized α-synuclein trafficking and transcytosis across brain endothelial cells via Rab7-decorated carriers
- PMID: 35637478
- PMCID: PMC9150364
- DOI: 10.1186/s12987-022-00334-y
Polarized α-synuclein trafficking and transcytosis across brain endothelial cells via Rab7-decorated carriers
Abstract
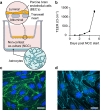
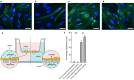
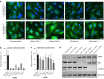
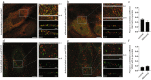
Parkinson's disease is mainly caused by aggregation of α-synuclein (α-syn) in the brain. Exchange of α-syn between the brain and peripheral tissues could have important pathophysiological and therapeutic implications, but the trafficking mechanism of α-syn across the blood brain-barrier (BBB) remains unclear. In this study, we therefore investigated uptake and transport mechanisms of α-syn monomers and oligomers across an in vitro BBB model system. Both α-syn monomers and oligomers were internalized by primary brain endothelial cells, with increased restriction of oligomeric over monomeric transport. To enlighten the trafficking route of monomeric α-syn in brain endothelial cells, we investigated co-localization of α-syn and intracellular markers of vesicular transport. Here, we observed the highest colocalization with clathrin, Rab7 and VPS35, suggesting a clathrin-dependent internalization, preferentially followed by a late endosome retromer-connected trafficking pathway. Furthermore, STED microscopy revealed monomeric α-syn trafficking via Rab7-decorated carriers. Knockdown of Caveolin1, VPS35, and Rab7 using siRNA did not affect monomeric α-syn uptake into endothelial cells. However, it significantly reduced transcytosis of monomeric α-syn in the luminal-abluminal direction, suggesting a polarized regulation of monomeric α-syn vesicular transport. Our findings suggest a direct role for Rab7 in polarized trafficking of monomeric α-syn across BBB endothelium, and the potential of Rab7 directed trafficking to constitute a target pathway for new therapeutic strategies against Parkinson's disease and related synucleinopathies.
Keywords: Brain endothelial cells and blood brain barrier; Parkinson’s disease; Polarization trap; Rab7; Retromer; Transcytosis; α-synuclein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

