Impairment of neutrophil functions and homeostasis in COVID-19 patients: association with disease severity
- PMID: 35637483
- PMCID: PMC9149678
- DOI: 10.1186/s13054-022-04002-3
Impairment of neutrophil functions and homeostasis in COVID-19 patients: association with disease severity
Abstract
Background: A dysregulated immune response is emerging as a key feature of critical illness in COVID-19. Neutrophils are key components of early innate immunity that, if not tightly regulated, contribute to uncontrolled systemic inflammation. We sought to decipher the role of neutrophil phenotypes, functions, and homeostasis in COVID-19 disease severity and outcome.
Methods: By using flow cytometry, this longitudinal study compares peripheral whole-blood neutrophils from 90 COVID-19 ICU patients with those of 22 SARS-CoV-2-negative patients hospitalized for severe community-acquired pneumonia (CAP) and 38 healthy controls. We also assessed correlations between these phenotypic and functional indicators and markers of endothelial damage as well as disease severity.
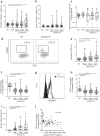
Results: At ICU admission, the circulating neutrophils of the COVID-19 patients showed continuous basal hyperactivation not seen in CAP patients, associated with higher circulating levels of soluble E- and P-selectin, which reflect platelet and endothelial activation. Furthermore, COVID-19 patients had expanded aged-angiogenic and reverse transmigrated neutrophil subsets-both involved in endothelial dysfunction and vascular inflammation. Simultaneously, COVID-19 patients had significantly lower levels of neutrophil oxidative burst in response to bacterial formyl peptide. Moreover patients dying of COVID-19 had significantly higher expansion of aged-angiogenic neutrophil subset and greater impairment of oxidative burst response than survivors.
Conclusions: These data suggest that neutrophil exhaustion may be involved in the pathogenesis of severe COVID-19 and identify angiogenic neutrophils as a potentially harmful subset involved in fatal outcome.
Keywords: Angiogenic neutrophils; COVID-19; Neutrophil; Oxidative burst; Vascular inflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

