Effects of cannabidiol on simulated driving and cognitive performance: A dose-ranging randomised controlled trial
- PMID: 35637624
- PMCID: PMC9716488
- DOI: 10.1177/02698811221095356
Effects of cannabidiol on simulated driving and cognitive performance: A dose-ranging randomised controlled trial
Abstract
Background: Cannabidiol (CBD), a major cannabinoid of Cannabis sativa, is widely consumed in prescription and non-prescription products. While CBD is generally considered 'non-intoxicating', its effects on safety-sensitive tasks are still under scrutiny.
Aim: We investigated the effects of CBD on driving performance.
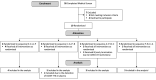
Methods: Healthy adults (n = 17) completed four treatment sessions involving the oral administration of a placebo, or 15, 300 or 1500 mg CBD in a randomised, double-blind, crossover design. Simulated driving performance was assessed between ~45-75 and ~210-240 min post-treatment (Drives 1 and 2) using a two-part scenario with 'standard' and 'car following' (CF) components. The primary outcome was standard deviation of lateral position (SDLP), a well-established measure of vehicular control. Cognitive function, subjective experiences and plasma CBD concentrations were also measured. Non-inferiority analyses tested the hypothesis that CBD would not increase SDLP by more than a margin equivalent to a 0.05% blood alcohol concentration (Cohen's dz = 0.50).
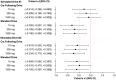
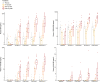
Results: Non-inferiority was established during the standard component of Drive 1 and CF component of Drive 2 on all CBD treatments and during the standard component of Drive 2 on the 15 and 1500 mg treatments (95% CIs < 0.5). The remaining comparisons to placebo were inconclusive (the 95% CIs included 0 and 0.50). No dose of CBD impaired cognition or induced feelings of intoxication (ps > 0.05). CBD was unexpectedly found to persist in plasma for prolonged periods of time (e.g. >4 weeks at 1500 mg).
Conclusion: Acute, oral CBD treatment does not appear to induce feelings of intoxication and is unlikely to impair cognitive function or driving performance (Registration: ACTRN12619001552178).
Keywords: Cannabidiol; cognition; driving simulation; medicinal cannabis; psychomotor.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: D.M., A.S.S., P.T.D., R.C.K. and I.S.M. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics. I.S.M. also acts as a consultant to Kinoxis Therapeutics and is an inventor on several patents relating to novel cannabinoid therapeutics. The other authors have no conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

