Water-Stable Carborane-Based Eu3+/Tb3+ Metal-Organic Frameworks for Tunable Time-Dependent Emission Color and Their Application in Anticounterfeiting Bar-Coding
- PMID: 35637791
- PMCID: PMC9136944
- DOI: 10.1021/acs.chemmater.2c00323
Water-Stable Carborane-Based Eu3+/Tb3+ Metal-Organic Frameworks for Tunable Time-Dependent Emission Color and Their Application in Anticounterfeiting Bar-Coding
Abstract
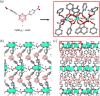
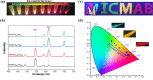
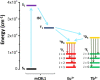
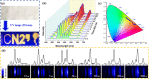
Luminescent lanthanide metal-organic frameworks (Ln-MOFs) have been shown to exhibit relevant optical properties of interest for practical applications, though their implementation still remains a challenge. To be suitable for practical applications, Ln-MOFs must be not only water stable but also printable, easy to prepare, and produced in high yields. Herein, we design and synthesize a series of m CB-Eu y Tb 1-y (y = 0-1) MOFs using a highly hydrophobic ligand mCBL1: 1,7-di(4-carboxyphenyl)-1,7-dicarba-closo-dodecaborane. The new materials are stable in water and at high temperature. Tunable emission from green to red, energy transfer (ET) from Tb3+ to Eu3+, and time-dependent emission of the series of mixed-metal m CB-Eu y Tb 1-y MOFs are reported. An outstanding increase in the quantum yield (QY) of 239% of mCB-Eu (20.5%) in the mixed mCB-Eu0.1Tb0.9 (69.2%) is achieved, along with an increased and tunable lifetime luminescence (from about 0.5 to 10 000 μs), all of these promoted by a highly effective ET process. The observed time-dependent emission (and color), in addition to the high QY, provides a simple method for designing high-security anticounterfeiting materials. We report a convenient method to prepare mixed-metal Eu/Tb coordination polymers (CPs) that are printable from water inks for potential applications, among which anticounterfeiting and bar-coding have been selected as a proof-of-concept.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. 10.1038/46248. - DOI
-
- Dhaka S.; Kumar R.; Deep A.; Kurade M. B.; Ji S.-W.; Jeon B.-H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. 10.1016/j.ccr.2018.10.003. - DOI
LinkOut - more resources
Full Text Sources
